MCQ (Previous Year Questions) - Organic Chemistry (Level 2) - JEE MCQ
20 Questions MCQ Test - MCQ (Previous Year Questions) - Organic Chemistry (Level 2)
Which of the following hydrocarbons has the lowest dipole moment? [JEE-2002]
Which of the following acids has the smallest dissociation constant ? [JEE-2002]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following represent the given mode of hybridisation sp2 – sp2 – sp – sp from left to right? [JEE-2003]
Maximum dipolemoment will be of [JEE-2003]
Product (A). The product A will be [JEE-2003]
[JEE-2004]
Correct order of acidic strength is
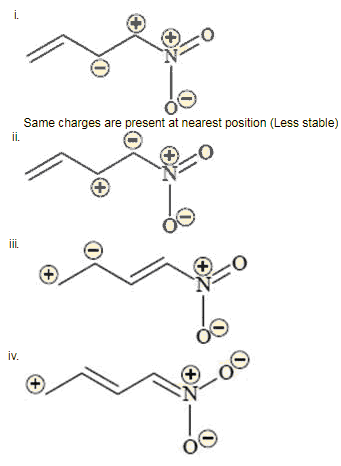
For 1-methoxy-1,3-butadiene, which of the following resonating structure is the least stable ? [JEE-2005]
When benzene sulfonic acid and p-nitrophenol are treated with NaHCO3, the gases released respectively are [JEE-2006]
(I) 1, 2 - dihydroxy beznene
(II) 1, 3 - dihydroxy benzene
(III) 1, 4 - dihydroxy benzene
(IV) Hydroxy benzene
The increasing order of boiling points of above mentioned alcohols is [JEE-2006]
Among the following, the least stable resonance structur is [JEE-2007]
Statement-1: p-Hydroxybenzoic acid has a lower boiling point then o-hydroxybenzoic acid.
Statement-2: o-Hydroxybenzoic acid has a intramoleculer hydrogen bonding. [JEE-2007]
Hyperconjugation involves overlap of the following orbitals [JEE-2008]
The correct stability order for the following species is [JEE-2008]
The correct acidity order of the following is [JEE-2009]
The correct stability order of the following resonance structures is [JEE-2009]
In the following carbocation; H/CH3 that is most likely to migrate to the positively charged carbon is [JEE-2009]
Among the following compounds, the most acidic is: [JEE-2011]
In Allen (C3H4), the type (s) of hybridisation of the carbon atoms is (are) [JEE-2012]
Which of the following molecules in pure from is (are) unstable at room temperature [JEE-2012]