MCQ (Previous Year Questions) - Thermochemistry (Level 2) - JEE MCQ
7 Questions MCQ Test - MCQ (Previous Year Questions) - Thermochemistry (Level 2)
Which of the following is not an endothermic reaction ? [JEE 1999]
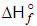 for CO2(g), CO(g) and H2O(g) are -393.5, -110.5 and -241.8 kJ mol-1respectively. The standard enthalpy change (in kJ) for the reaction [JEE 2000]
for CO2(g), CO(g) and H2O(g) are -393.5, -110.5 and -241.8 kJ mol-1respectively. The standard enthalpy change (in kJ) for the reaction [JEE 2000]
CO2 (g) + H2(g)  CO(g) + H2O (g) is
CO(g) + H2O (g) is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following reactions defines 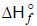 ? [JEE 2003]
? [JEE 2003]
The bond energy (in kcal mol-1) of a C - C single bond is approximately [JEE 2010]
The species which by definition has zero standard molar enthalpy of formation at 298 K is [JEE 2010]
Using the data provided, calculate the multiple bond energy (kJ mol-1) of a CC bond in C2H2. That energy is (take the bond energy of a C_H bond as 350 kJ mol-1) [JEE 2012]
2C(s) + H2(g) C2H2(g) ΔH = 225 kJ mol-1
2C(s) 2C(g) ΔH = 1410 kJ mol-1
H2(g) 2H(g) ΔH = 330 kJ mol-1
The standard enthalpies of formation of CO2(g), H2O(l) and glucose(s) at 25ºC are -400 kJ/mol, -300 kJ/mol and -1300 kJ/mol, respectively. The standard enthalpy of combustion per gram of glucose at 25ºC is [JEE Advance 2013]

















