MOCK TEST - 1 - IIT JAM MCQ
30 Questions MCQ Test - MOCK TEST - 1
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In the MO configuration of XeF2, how many pairs are present in BMO, NBO, ABMO, respectively:
Which of the following statement is correct about the following compounds
(Et3P)2PtCl2 and (Et2S)2PtCl2
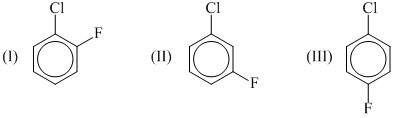
Arrange the following compounds in decreasing order of dipole moment:
KCl can be used in salt bridge as electrolyte in which of the following cells:
Given that (at T = 298 K)
Cu(s) | Cu2+ (1.0 M) || Ag+ (1.0 M) | Ag(s) E°cell = 0.46 V
Zn(s) | Zn2+ (1.0 M) || Cu2+ (1.0 M) | Cu(s) E°cell = 1.10 V
Then Ecell for
Zn | Zn2+ (1.0 M) || Ag+ (1.0 M) | Ag at 298 K will be
The standard emf of the cell, Cd(s) | CdCl2(aq) (0.1 M) || AgCl(s) | Ag(s) in which the cell reaction, is, 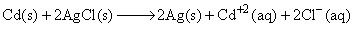 is 0.6915 V at 0°C and 0.6753 V at 25°C. The ΔH° of the reaction at 25°C is:
is 0.6915 V at 0°C and 0.6753 V at 25°C. The ΔH° of the reaction at 25°C is:
Aqueous solution of Na2SO4 containing a small amount of HPh is electrolyzed using Pt-electrodes. The color of the solution after some time will:
A current is passed through 2 voltameters connected in series. The first voltameter contains XSO4(aq.) and second has Y2SO4(aq.). The relative atomic masses of X and Y are in the ratio of 2:1. The ratio of the mass of X liberated to the mass of Y liberated is:
A resistance of 50Ω is registered when two electrodes are suspended into a beaker containing a dilute solution of a strong electrolyte such that exactly half of half of the them are submerged into solution as shown in figure. If the solution is diluted by adding pure water (negligible conductivity) so as to just completely submerge the electrodes, the new resistance of offered by the solution would be:
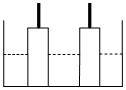
The conductivity of a saturated solution of BaSO4 is 3.06 × 10–6 ohm–1 cm–1 and its equivalent conductance is 1.53 ohm–1 cm2 equiv–1. The Ksp for BaSO4 will be:
The standard reduction potentials for Zn2+/Zn, Ni2+/Ni and Fe2+/Fe are –0.76, –0.23 and –44 V respectively. The reaction X + Y2+ → X2+ + Y will be spontaneous when:
Choose the correct option for carbonyl fluoride with respect to bond angle and bond length:
Which one among the following chlorides is dissociated to the least extent in aqueous solution?
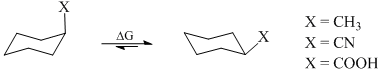
The correct order of magnitude of ‘ΔG value’ for the given substituents in cyclohexane derivatives is:
AgOH was added to an aqueous HCl solution gradually and the conductivity of the solution was measured. The plot of conductance  versus the volume of AgOH is:
versus the volume of AgOH is:
The total number of acyclic structural and optical isomers possible for a hydrocarbon of molecular formula C7H16
Arrange the following conformation in increasing order of their stability:
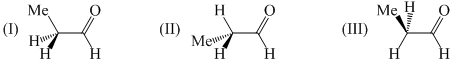
The correct order of axial conformation in the 2-substituted Cyclohexanone in CDCl3 is:
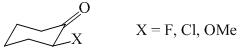


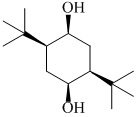
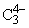 has:
has: