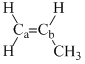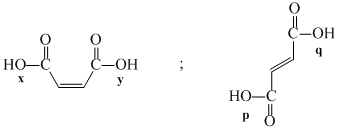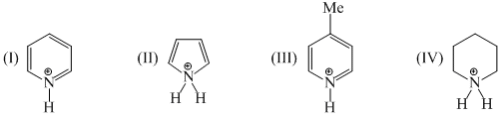MOCK TEST-III - IIT JAM MCQ
30 Questions MCQ Test - MOCK TEST-III
The wavelength associated with a particle in one-dimensional box of length L is (n refers to the quantum number)
The electronic ground state term for the chromium ion in [Cr(CN)6]4– is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The linear momentum of a particle described by the wave function e–ikx is:
Of the following inequalities, the criterion/criteria for spontaneity of a chemical reaction is/are:
(I) (ΔG)T, P < 0 (II) (ΔU)S, V > 0 (III) (ΔS)U, V > 0
The experimental ionization energies of hydrogen and helium atoms in their ground states are respectively, 13.6 eV and 24.6 eV. The ground state energy of helium atom, in eV, is:
Which among the following pairs of reactions has the most favourable equilibrium constant?
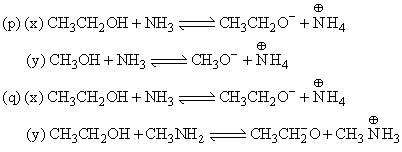
The correct order of acidic nature of the following compounds is:
(I)  (II)
(II) 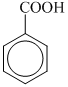 (III)
(III) 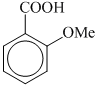 (IV)
(IV) 
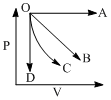
The two lines A and B shown in the graph plot the de-Broglie wave length (λ) as function of 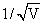 (V is the accelerating potential) for two particles having the same charge. Which of the two represents the particle of heavier mass:
(V is the accelerating potential) for two particles having the same charge. Which of the two represents the particle of heavier mass:
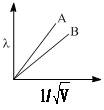
Consider the following radial distribution function diagrams. Which of the following has correct matching of curve and orbitals:
ψ = Nr (6–Zr)–Zr/3 cosθ, is a proposed hydrogenic wave function, where
Z = Atomic number
r = radial distance from the nucleus, θ = azimuthal angles, N is a constant. The incorrect statement about ψ is:
One mole of a non-ideal gas undergoes a change of state (2.0 atm, 3.0 L, 95 K)  (4.0 atm, 5.0 L, 245 K) with a change in internal energy, ΔU = 30.0 L atm. The change in enthalpy (ΔH) of the process in L atm is:
(4.0 atm, 5.0 L, 245 K) with a change in internal energy, ΔU = 30.0 L atm. The change in enthalpy (ΔH) of the process in L atm is:
The electronic configurations that have orbital angular momentum contribution in an octahedral environment are:
For tetrahedral complexes, which always exhibit high spin states, the maximum CFSE (Crystal Field Stabilization energy) is:
The plot of  versus T (where
versus T (where 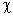 is molar magnetic susceptibility and T is the temperature) for a paramagnetic complex which strictly follows Curie equation is:
is molar magnetic susceptibility and T is the temperature) for a paramagnetic complex which strictly follows Curie equation is:
The spin-only (μS) and spin plus orbital (μS+L) magnetic moment of [CrCl6]3– are:
When one mole of monoatomic ideal gas at TK undergoes adiabatic change under a constant external pressure of 1 atm changes volume from 1 litre to 2 litre. The final temperature in Kelvin would be:
For a given reaction, ΔH = 35.5 kJ mol–1 and ΔS = 83.6 JK–1 mol–1. The reaction is spontaneous at (Assume that ΔH and ΔS do not vary with temperature):
The 2s-orbital of H-atom has a radial node at 2a0 because ψ2s is proportional to:
Consider a particle in its ground state confined to a one-dimensional box in the interval (0, 8). The probability of finding it between 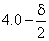 and
and 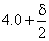 is close to (
is close to ( 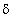 is sufficiently small so that the wave function can be taken as a constant in this interval)
is sufficiently small so that the wave function can be taken as a constant in this interval)
Which of the functions below is a common eigen function of 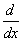 and
and 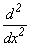 operators?
operators?
Arrange the following compounds in order of Ca—Cb bond strength:
(I) 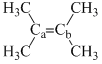 (II)
(II) 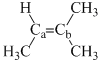
(III) 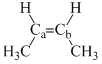 (IV)
(IV)