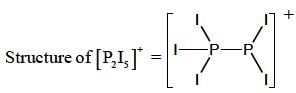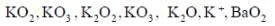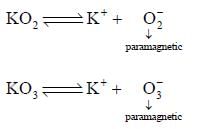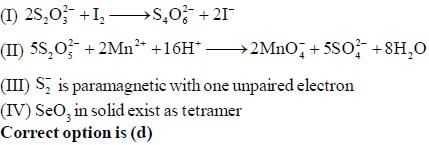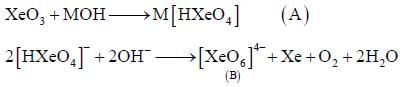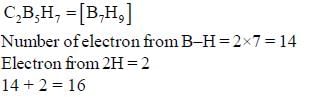Main Group Elements - GATE Chemistry MCQ
20 Questions MCQ Test GATE Chemistry Mock Test Series - Main Group Elements
Select the energy band which represent the electrical conductivity in direction parallel to chain in graphite
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Correct order of metal ion regarding logβ value for [M-crown-6]+ in gas phase is
Reaction of S4N4 with metallic potassium or sodium leads to formation of

31P NMR spectrum of cation shows _____doublet of equal intensity
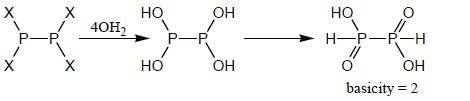
Hydrolysis of P2X4 produces an acid which have basicity _________
Electrophilic substitution reaction of terminal H atom attached to B in closo-1, 2 C2B10H12 follow the sequence
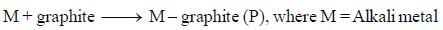
The correct statement regarding P is/are
(I) electrical conductivity decreases with increase in temperature
(II) M-graphite compounds are coloured.
(III) Mechanism of conductivity in M-graphite complexes are similar to conductivity in n-type semiconductors.
(IV) In product planarity and delocalization of layers are retained.
Match Column-A (species) with Column-B (properties related to species)

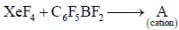
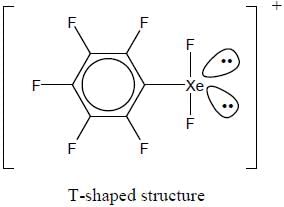
Correct statement regarding A is/are
(1) It has T-shaped structure
(2) 129Xe NMR spectrum show a triplet
(3) 19F NMR spectrum show four signal
(4) It has TBP geometry
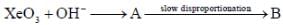
Correct statement regarding A and B are
(1) A is very strong oxidising agent
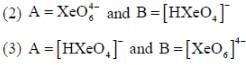
(4) B is strong oxidising agent.
Correct statement regarding C60 is/are
(1) It has Ih point group
(2) Its satisfies IPR
(3) It has two kind of C–C bond length
(4) It is more reactive than graphite
Number of framework electron in carbonanes C2B5H7 is ________
|
18 docs|37 tests
|
|
18 docs|37 tests
|


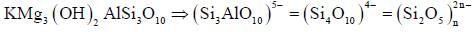
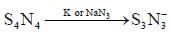
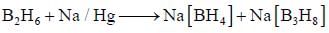
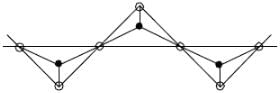
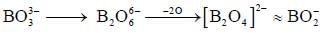
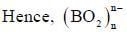
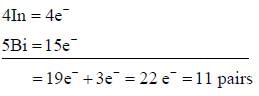
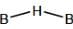 bond also called Banana Bond.
bond also called Banana Bond.