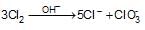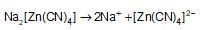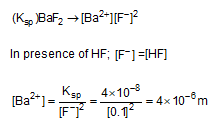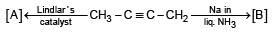Mock Test - 1 - Class 12 MCQ
30 Questions MCQ Test - Mock Test - 1
How many atoms of He would occupy the same volume as molecules of N2O4 , under similar temperature and pressure conditions?
Three gases X, Y and Z has the van der Waals’ gas constant values ‘a’ as 1.42, 3.85 and 2.26 litre2 atm mol-2. The tendency of getting liquified is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
1 mole of chlorine gas in hot and concentrated alkaline medium disproportionates to
In which of the following the cations occupy alternate tetrahedral voids in a cubic close packed arrangement ?
What will be the van’t Hoff factor of the electrolyte in a solution having completely ionised complex salt sodium tetracyanozincate (II) ?
The solubility product has been recorded as 4 * 10-8 for BaF2. What will the solubility of BaF2 in a 0.1M HF solution ?
Some electrode potential data is given below
Fe3+ + e- → Fe2+ ; E0 = 0.77V
Zn2+ + 2e- → Zn; E0 = -0.76V
Br2 + 2e- → 2Br-; E0 = 1.08V
Ag+ + e- → Ag; E0 = 0.80V
According to the data given above, the reducing ability of various species can be arranged as
Suggest the set of reagents that can bring about the following conversion

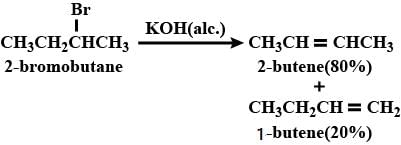
Elimination of bromine from 2-bromo butane results in the formation of:
Which one of the following reactions examplify the oxidising property of hydrogen peroxide ?
The multiplicity of electrons in 3d5 electronic configuration is equal to
KF combines with HF to form KHF2. The compound contains the species
If the intermolecular forces vanish away, the volume occupied by the molecules contained in 4.5 kg water at STP will be
If uncertainty in position of electron is zero, the uncertainty in its momentum would be
During developing of an exposed camera film, one step involves the following reaction,

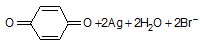
Which of the following best describes the role of hydroquinol, it acts as
The data given below are for vapour phase reactions at constant pressure
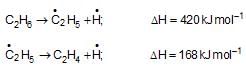
The enthalpy change for the reaction
For the following electrochemical cell
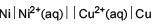
EMF of the cell will increase
Consider the following statements
(i) First order reaction completes in infinite time. This true and a basic of reaction kinetics
(ii) Average life is independent of concentration for first order reactions t = 1/λ
(iii) t75% = 1.5 t1/2 for zero order reaction as there is linear dependence
(iv) t75% = 2t1/2 for first order reaction as there is exponential dependence
Correct among the following are
Which of the following will be oxidised by HIO4?
(i) R - CO - CO - R
(ii) R - CO - CHOH - R
(iii) R - CHOH - CH2 - CHOH - R
(iv) R - CHOH - CHOH - R