NEET Part Test - 7 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series 2025 - NEET Part Test - 7
During which stage of prophase I the crossing over takes place?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which phase of meiosis in animal cells is shown in figure given below?
As they release hydrolase that digest old andd damaged cells, the term suicide bags is aptly used by cell biologists for
In most fungi the structural polysaccharide of cell wall is ------
Which of the following nitrogenous base produces nucleoside only with ribose sugar?
An irreversible or permanent increase in size, mass or volume of a cell, organ or organism is called as ________.
_________ indudes all the changes that an organism undergoes during its life cycle, from seed germination to senescence.
Read the following statements regarding arithmetic growth and select the correct answer.
(i) Rate of growth is constant.
(ii) One daughter cell remains meristematic while the other one differentiates and matures.
(iii) Mathematical expression is Lt =L0 +rt.
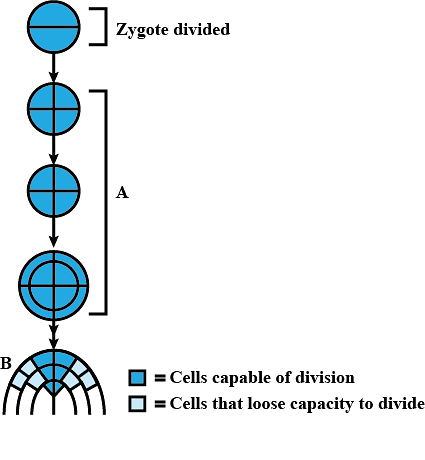
The given figure shows development of an embryo that undergoes two phases A and B. Select the correct option regarding it.
In the systemic circulation, the blood vessel that carries blood from the intestine to the liver is named:
The pre-hypertension blood pressure value is a measurement between:
Which of the following statements regarding the structure of microsporangium are correct?
(i) Microsporangium is generally surrounded by four wall layers-epidermis, endothecium, middle layers, and tapetum.
(ii) Outer three layers perform functions of protection and dehiscence of anthers.
(iii) Cells of tapetum undergo meiosis and produce microspore tetrads.
A dithecous anther consists of ____(i)_______microsporangia, _____(ii)_______ in each lobe.
Bt corn has been made resistant from corn borer disease by introduction of the gene
Cry genes can be transferred as they are present in a bacteria in______.
A collection of methods that allows correction of a gene that has been diagnosed in child/embryo is called _________
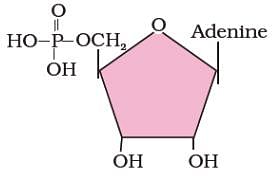
Identify the biomolecule shown in the structural diagram given below.
|
1 videos|26 docs|111 tests
|
|
1 videos|26 docs|111 tests
|


















