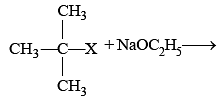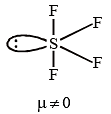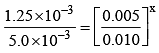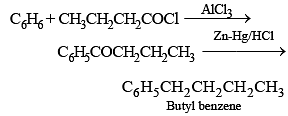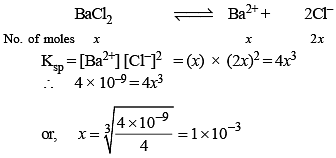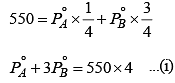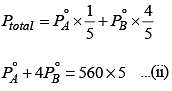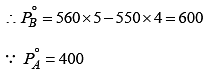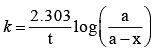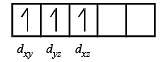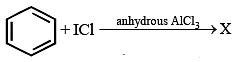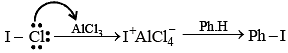NEET Practice Test - 15 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series 2025 - NEET Practice Test - 15
In Williamson synthesis of mixed ether having a primary and a tertiary alkyl group if tertiary halide is used, then :
Nitration of aniline also gives m-nitroaniline, in strong acidic medium because
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The orbital having m = – 2 should not be present in the following sub-shell
The correct order regarding the electronegativity of hybrid orbitals of carbon is
Which of the following would have a permanent dipole moment?
The initial rates of reaction
3A + 2B + C ⟶ Products, at different initial concentrations are given below:
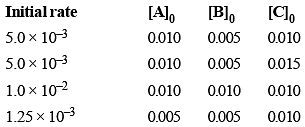
The order with respect to the reactants, A, B and C are respectively
The conduct of a salt solution (AB) measured by two parallel electrodes of area 100 cm2 separated by 10 cm was found to be 0.0001 W–1. If volume enclosed between two electrode contain 0.1 mole of salt, what is the molar conductivity (S cm2 mol–1) of salt at same concentration :
Addition of phosphate fertilizers to water bodies causes:
The reaction conditions leading to the best yields of C2H5Cl are :
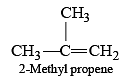
Which of the following would not give 2-phenylbutane as the major product in a Fried Crafts alkylation of benzene?
Solubility product of BaCl2 is 4 × 10–9 mol/L. Its solubility would be :
Which of the following statements about kinetic energy (K.E.) is true?
What is the weight of oxygen required for the complete combustion of 2.8 kg of ethylene ?
The product formed in the reaction of SOCl2 with white phosphorous is
Two liquids X and Y form an ideal solution. At 300 K, the vapor pressure of the solution containing 1 mol of X and 3 mol of Y is 550 mmHg.
At the same temperature, if 1 mol of Y is further added to this solution, vapor pressure of the solution increases by 10 mmHg. Vapor pressure ( in mmHg) of X and Y in their pure states will be, respectively:
Which of the following reactions cannot proceed by SN1 mechanism ?
Which one of the following pairs of species have the same bond order?
For a first order reaction the rate constant is 6.909 min–1. The time taken for 75% conversion in minutes is
[Cr(H2O)6]Cl3 (at. no. of Cr = 24) has a magnetic moment of 3.83 B. M. The correct distribution of 3d electrons in the chromium of the complex is
Arrange (I) Ce3+, (II) La3+ (III) Pm3+ and (IV) Yb3+ in increasing order of their ionic radii.
|
1 videos|26 docs|111 tests
|
|
1 videos|26 docs|111 tests
|