NVS PGT Chemistry Mock Test - 6 - NVS TGT/PGT MCQ
30 Questions MCQ Test NVS PGT Mock Test Series 2025 - NVS PGT Chemistry Mock Test - 6
The outermost shell of a ______ element has two electrons.
In a certain code, MONK is written as 53. How will TUTOR be written in that code?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following is/are the parts of an e-mail address?
Which command moves the turtle to another position or the screen without drawing a line?
In Microsoft-Word, what is the use of combination keys Ctrl + S?
The basic cause of a teacher`s failure in maintaining discipline is the lack of
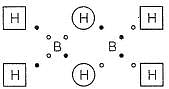
Bond order of (B— H) x and y bonds in diborane are respectively
The most and least reactive electrophiles respectively in a SN1 reaction are
Which one of the following aqueous solutions will exhibit highest boiling point ?
[AIEEE-2004]
Among the following sets, highest boiling points are of the species.
I. HF, HCI, HBr, HI
II. H2O, H2S, H2Se, H2Te
III. NH3,PH3, AsH3,SbH3
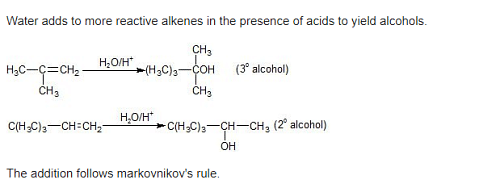
Acid catalyzed hydration of alkenes except ethene leads to the formation of –
[AIEEE-2005]
the oxidation number of sulphur in S8,S2F2,H2S respectively are
Elements of which group are known as ore forming elements?
Pyrolusite in MnO2 is used to prepare KMnO4. Steps are
Here, I and II are
For the reaction
A(g) + 2B(g)  C(g) + D(g); Kc = 1012 If the initial moles of A, B, C and D are 0.5, 1, 0.5 and 3.5 moles respectively in a one litre vessel. What is the equilibrium concentration of B ?
C(g) + D(g); Kc = 1012 If the initial moles of A, B, C and D are 0.5, 1, 0.5 and 3.5 moles respectively in a one litre vessel. What is the equilibrium concentration of B ?
Energy of the electron in nth orbit is given by E Wavelength of light required to excite an electron in an H-atom from level n = 1 to n = 2 will be (h = 6.62 x 10-34 J s ; c = 3.0 x 108ms -1)
[AIEEE 2012]
The complex [Co(NH3)6[Cr(C2O4)3] and [Cr(NH3)6[Co(C2O4)3] exhibit
Bond length of H— Cl is 1.2476 Thus, its dipole moment is
[Ti (H2O)6]3+ absorbs green and yellow region part of visible light. Then the transmitted colour of the compound is
Which of the following is an example of scaffolding?
The question below consists of a set of labeled sentences. Out of the four options given, select the most logical order of the sentences to form a coherent paragraph.
P: The climate has been changing drastically over the past years.
Q: If we don't correct the situation now, maybe we will never get a chance again.
R: Human activities itself are the main reason behind this.
S: The redressal of this problem is the biggest challenge of our generation.
|
30 tests
|