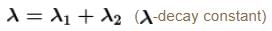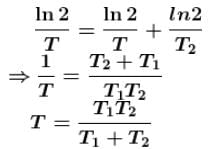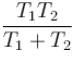Nuclear Physics MCQ Level – 1 - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - Nuclear Physics MCQ Level – 1
The binding energies of the nuclei Pn and Q2n are x and y joules. If 2x > y then the energy released in the reaction Pn + Pn → Q2n, will be
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
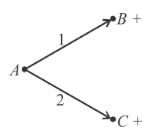
A radioactive element Th(84Po216) can undergo α and β are type of disintegration with half lives, T1 and T2 respectively. Then the half life of Th is .
The order of magnitude of the density of nuclear matter is :
The value of binding energy for 1H2, 2He4, 28Fe56, 92U235 are 2.22, 28.3, 492 and 1786 units respectively. The most stable nucleus is :
In equation 92U235 + 0n1 → 56Ba144 + 36Kr89 + X ; X is :
Masses of nucleus, neutron an protons are M, mn and mp respectively. If nucleus has been divided into neutrons and protons, then
In most stable nuclei neutron number N and proton number Z has the relation
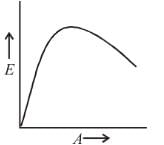
The graph between the binding energy per nucleon (E) and atomic mass number (A) is as :