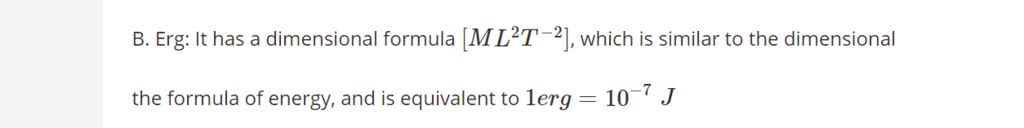Class 7 Exam > Class 7 Tests > Science Olympiad Class 7 > Olympiad Test: Heat And Temperature -1 - Class 7 MCQ
Olympiad Test: Heat And Temperature -1 - Class 7 MCQ
Test Description
15 Questions MCQ Test Science Olympiad Class 7 - Olympiad Test: Heat And Temperature -1
Olympiad Test: Heat And Temperature -1 for Class 7 2024 is part of Science Olympiad Class 7 preparation. The Olympiad Test: Heat And Temperature -1 questions and answers have been
prepared according to the Class 7 exam syllabus.The Olympiad Test: Heat And Temperature -1 MCQs are made for Class 7 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Olympiad Test: Heat And Temperature -1 below.
Solutions of Olympiad Test: Heat And Temperature -1 questions in English are available as part of our Science Olympiad Class 7 for Class 7 & Olympiad Test: Heat And Temperature -1 solutions in
Hindi for Science Olympiad Class 7 course. Download more important topics, notes, lectures and mock
test series for Class 7 Exam by signing up for free. Attempt Olympiad Test: Heat And Temperature -1 | 15 questions in 30 minutes | Mock test for Class 7 preparation | Free important questions MCQ to study Science Olympiad Class 7 for Class 7 Exam | Download free PDF with solutions
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 1
Olympiad Test: Heat And Temperature -1 - Question 2
One glass of water at 40ºC is mixed with another glass of water at 60ºC. The temperature of mixture will be:
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Olympiad Test: Heat And Temperature -1 - Question 3
The degree of hotness or coldness of a body is measured by its
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 3
Olympiad Test: Heat And Temperature -1 - Question 4
Mercury is an ideal liquid used in a thermometer because
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 4
Olympiad Test: Heat And Temperature -1 - Question 5
At low temperatures ______ type of thermometers is used
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 5
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 6
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 7
Olympiad Test: Heat And Temperature -1 - Question 8
When we touch a steel rod and a paper simultaneously, we feel that the rod is colder because
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 8
Olympiad Test: Heat And Temperature -1 - Question 9
The lower fixed point on the Celsius scale is
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 9
Olympiad Test: Heat And Temperature -1 - Question 10
In the Celsius scale, the upper fixed point is
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 10
Olympiad Test: Heat And Temperature -1 - Question 11
In which of the following, chemical energy is converted into heat energy.
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 11
Olympiad Test: Heat And Temperature -1 - Question 12
Handles of cooking utensils should be made of materials that:
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 12
Olympiad Test: Heat And Temperature -1 - Question 13
Which of the following is a good conductor of heat?
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 13
Olympiad Test: Heat And Temperature -1 - Question 14
Which of the following is a bad conductor of heat?
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 14
Detailed Solution for Olympiad Test: Heat And Temperature -1 - Question 15
|
34 videos|88 docs|54 tests
|
Information about Olympiad Test: Heat And Temperature -1 Page
In this test you can find the Exam questions for Olympiad Test: Heat And Temperature -1 solved & explained in the simplest way possible.
Besides giving Questions and answers for Olympiad Test: Heat And Temperature -1, EduRev gives you an ample number of Online tests for practice
|
34 videos|88 docs|54 tests
|
Download as PDF