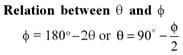Origin Of Quantum Mechanics MCQ Level – 1 - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - Origin Of Quantum Mechanics MCQ Level – 1
If first excitation potential of a hydrogen-like atom is V eV, then the ionization energy of this atom will be :
Linear momenta of a proton and an electron are equal. Relative to an electron
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The wavelength of a photon and the de-Broglie wavelength of an electron and uranium atom are identical. Which one of them will have highest kinetic energy
The energy E and momentum p of photon is given by E = hv and p = h/λ, the velocity of photon will be :
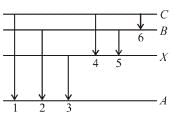
In the figure six lines of emission spectrum are shown. Which of them will be absent in the absorption spectrum.
A charge particle q0 of mass m0 is projected along the y-axis at t = 0 from origin with a velocity V0. If uniform electric field E0 also exists along the x-axis, then the time at which de-Broglie wavelength of the particle becomes half of the initial value is :
What voltage must be applied to an electron microscope to produce electrons of 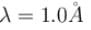
In X-rays production an electron accelerated with voltage V strikes a metal target. For which of the following voltages X-rays of minimum wavelength will be produced :
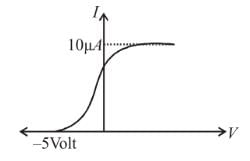
In the photoelectric, if we use a monochromatic light, the I-V curve is as shown. If work function of the metal is 2eV, estimate the power of light used. (Assume efficiency of photo emission = 10–3 % i.e. number of photoelectrons emitted are 10–3 % of number of photons incident on metal.)
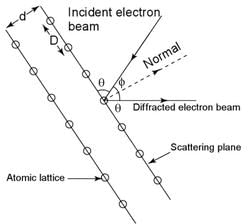
In Davisson-Germer experiment the relation between the angle of diffraction θ and the grazing angle φ is.


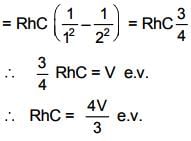
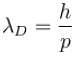
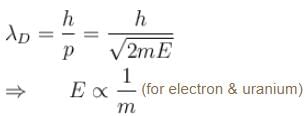

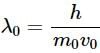
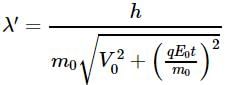
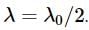
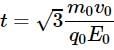
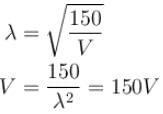
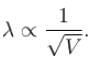

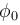 is work function)
is work function)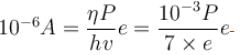 (η is photo emission efficiency)
(η is photo emission efficiency)