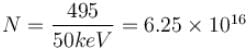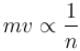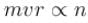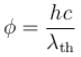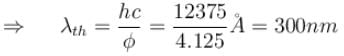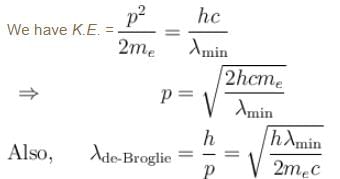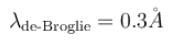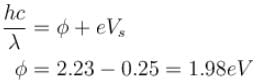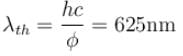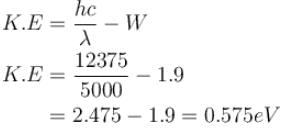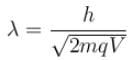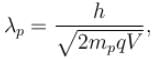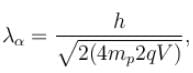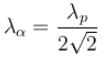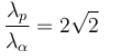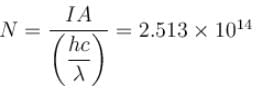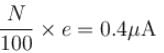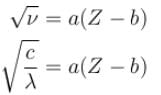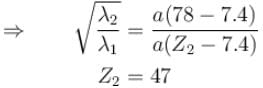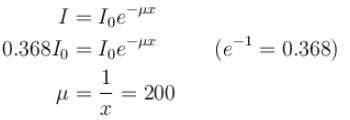Origin Of Quantum Mechanics NAT Level – 1 - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - Origin Of Quantum Mechanics NAT Level – 1
An X-ray tube operating at 50kV converts 1% energy in the form of X-ray. If the amount of heat produced is 495 watts, then the number of electrons colliding with the target per second is 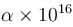 . Find the value of α.
. Find the value of α.
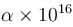 . Find the value of α.
. Find the value of α.In a hydrogen atom following Bohr's postulates, the product of linear momentum and angular momentum is proportional to (n)x where n is the orbit number. Then x is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Work function for aluminum metal is 4.125 eV. For photo electric effect from aluminum metal, the cut off wavelength (in nm) will be.
If the short wavelength limit of the continuous spectrum coming out of a coolidge tube is 10Å, then the de-Broglie wavelength (in Å)of the electrons reaching the largest metal in the coolidge tube is approximately.
Yellow light of 557 nm wavelength is incident on a cesium surface. It is found that no photo-electron flow in the circuit when the cathode anode voltage drops below 0.25V. Then the threshold wavelength (in nm) for photoelectric effect from cesium is.
Light of wavelength 5000Å falls on a sensitive plate with photoelectric work function of 1.9 eV. The kinetic energy (in eV) of the emitted photo-electron will be.
The ratio of de-Broglie wavelength of a proton and an α-particle accelerated through the same potential difference is:
When light of intensity 1 W/m2 and wavelength 5 × 10–7 m is incident on a surface, it is completely absorbed by the surface. If 100 photons emit one electron and area of the surface is 1 cm2, then the photoelectric current (in µA) will be.
The wavelength of Ln line in X-rays spectrum of 78Pt is 1.32 Å. The wavelength of Ln line in X-rays spectrum of another unknown element is 4.17Å. If screening constant for La line is 7.4, then atomic number of the unknown element is :
The intensity of an X-ray beam reduces to 36.8% of its initial intensity after traversing a gold film of thickness 5 × 10–3 m. Its absorption coefficient (in m–1) is :