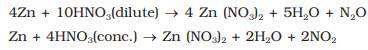P Block MCQ - 2 (Advanced) - JEE MCQ
15 Questions MCQ Test - P Block MCQ - 2 (Advanced)
Stability of monovalent and trivalent cations of Ga, In, lie in following sequence :
Orthoboric acid (H3BO3) and metaboric acid (HBO2) differ in respect of :
Decomposition of oxalic acid in presence of conc. H2SO4 gives :
The metals which produce hydrogen only with very dilute nitric acid are :
Which of the following form oxychlorides as precipitate on hydrolysis ?
Passage
When a mixture of sodium carbonate and calcium carbonate is fused with silica at 1500ºC, a liquid consisting silicates of sodium and calcium is formed. When this liquid is cooled, it becomes viscous and eventually ceases to flow. It becomes solid and called glass. By varying the proportions of the three basic ingredients and by adding other substances, the properties of glass can be altered. An approximate formula for ordinary glass may be given as,
R2O.MO.6SiO2
Where R = Na or K and M = Ca, Ba, Zn and Pb.
SiO2 may be replaced by Al2O3, B2O3, P2O5.
Coloured glasses are obtained by adding certain metallic oxides or salts in the fused mass. Class is attacked by HF and this property is used to make marking on the glass. This is known as etching.
The glass if cooled rapidly becomes brittle and fragile. The articles of glass are cooled neither very slowly nor very rapidly. The articles are cooled gradually. This process is termed annealing.
Q.
Annealing is the best described as:
When a mixture of sodium carbonate and calcium carbonate is fused with silica at 1500ºC, a liquid consisting silicates of sodium and calcium is formed. When this liquid is cooled, it becomes viscous and eventually ceases to flow. It becomes solid and called glass. By varying the proportions of the three basic ingredients and by adding other substances, the properties of glass can be altered. An approximate formula for ordinary glass may be given as,
R2O.MO.6SiO2
Where R = Na or K and M = Ca, Ba, Zn and Pb.
SiO2 may be replaced by Al2O3, B2O3, P2O5.
Coloured glasses are obtained by adding certain metallic oxides or salts in the fused mass. Class is attacked by HF and this property is used to make marking on the glass. This is known as etching.
The glass if cooled rapidly becomes brittle and fragile. The articles of glass are cooled neither very slowly nor very rapidly. The articles are cooled gradually. This process is termed annealing.
Q.
A blue colour can be imparted to glass by use of :
When a mixture of sodium carbonate and calcium carbonate is fused with silica at 1500ºC, a liquid consisting silicates of sodium and calcium is formed. When this liquid is cooled, it becomes viscous and eventually ceases to flow. It becomes solid and called glass. By varying the proportions of the three basic ingredients and by adding other substances, the properties of glass can be altered. An approximate formula for ordinary glass may be given as,
R2O.MO.6SiO2
Where R = Na or K and M = Ca, Ba, Zn and Pb.
SiO2 may be replaced by Al2O3, B2O3, P2O5.
Coloured glasses are obtained by adding certain metallic oxides or salts in the fused mass. Class is attacked by HF and this property is used to make marking on the glass. This is known as etching.
The glass if cooled rapidly becomes brittle and fragile. The articles of glass are cooled neither very slowly nor very rapidly. The articles are cooled gradually. This process is termed annealing.
Q.
A special type of glass which contains cerium oxide and does not allow the passage of ultraviolet rays.
This glass is used for making lenses. The glass is colled: