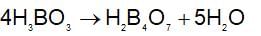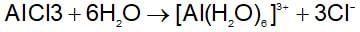Class 12 Exam > Class 12 Tests > P-Block - Class 12 MCQ
P-Block - Class 12 MCQ
Test Description
30 Questions MCQ Test - P-Block
P-Block for Class 12 2024 is part of Class 12 preparation. The P-Block questions and answers have been prepared
according to the Class 12 exam syllabus.The P-Block MCQs are made for Class 12 2024 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for P-Block below.
Solutions of P-Block questions in English are available as part of our course for Class 12 & P-Block solutions in
Hindi for Class 12 course.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free. Attempt P-Block | 40 questions in 45 minutes | Mock test for Class 12 preparation | Free important questions MCQ to study for Class 12 Exam | Download free PDF with solutions
Detailed Solution for P-Block - Question 1
Detailed Solution for P-Block - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Detailed Solution for P-Block - Question 3
Detailed Solution for P-Block - Question 4
Detailed Solution for P-Block - Question 5
Detailed Solution for P-Block - Question 6
Detailed Solution for P-Block - Question 7
P-Block - Question 9
Aluminum is more reactive than iron, but aluminium is less easily corroded than iron because:
Detailed Solution for P-Block - Question 9
Detailed Solution for P-Block - Question 10
Detailed Solution for P-Block - Question 11
Detailed Solution for P-Block - Question 12
P-Block - Question 13
Aqueous ammonia is used as a precipitating reagent for AI3+ ions as AI(OH)3 rather than aqueous NaOH, because:
Detailed Solution for P-Block - Question 13
Detailed Solution for P-Block - Question 14
Detailed Solution for P-Block - Question 15
Detailed Solution for P-Block - Question 16
Detailed Solution for P-Block - Question 17
P-Block - Question 18
The stability of +1 oxidation state among AI,Ga,In and TI increases in the sequence:
Detailed Solution for P-Block - Question 18
P-Block - Question 19
Boric acid is an acid because its molecule:
H3BO3 is monobasic Lewis acid and accept OH- from H2O forming one proton or H+.
Detailed Solution for P-Block - Question 19
Detailed Solution for P-Block - Question 20
P-Block - Question 21
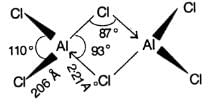
Aluminium chloride exists as dimer,AI2CI6 in soild state as well as in solution of non-polar solvent such as benzene. When dissolved in water it gives:
Detailed Solution for P-Block - Question 21
P-Block - Question 22
The state of hybridisation of boron and oxygen atoms in boric acid (H3BO3) are respectively:
Detailed Solution for P-Block - Question 22
P-Block - Question 23
Graphite is good conductor of current but diamond is non-conductor because:
Detailed Solution for P-Block - Question 23
Detailed Solution for P-Block - Question 24
Detailed Solution for P-Block - Question 25
Detailed Solution for P-Block - Question 26
Detailed Solution for P-Block - Question 27
Detailed Solution for P-Block - Question 28
Detailed Solution for P-Block - Question 29
Detailed Solution for P-Block - Question 30
View more questions
Information about P-Block Page
In this test you can find the Exam questions for P-Block solved & explained in the simplest way possible.
Besides giving Questions and answers for P-Block, EduRev gives you an ample number of Online tests for practice
Download as PDF