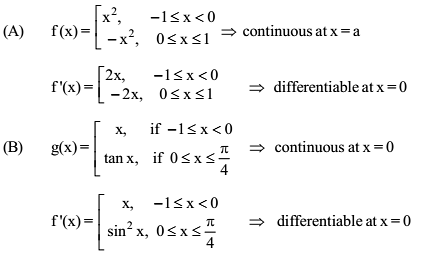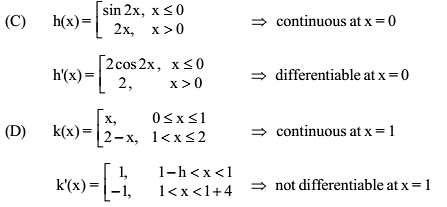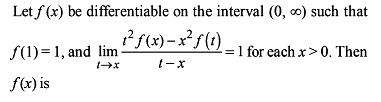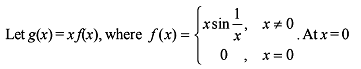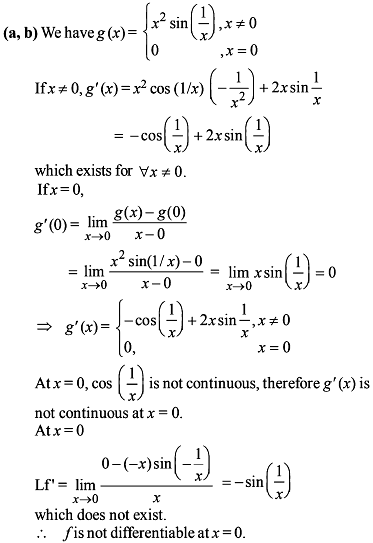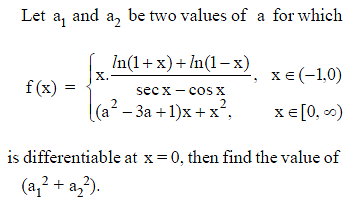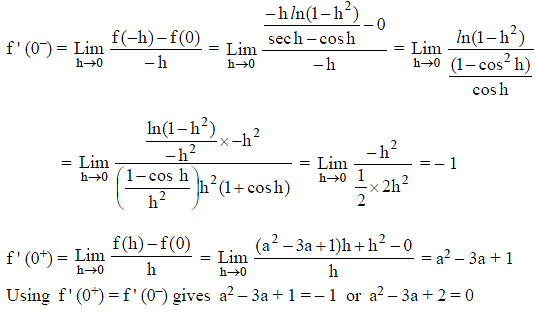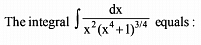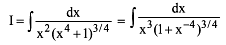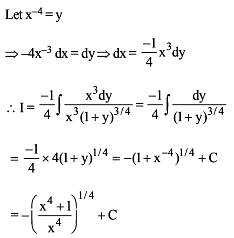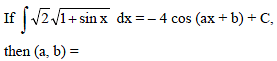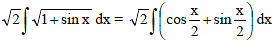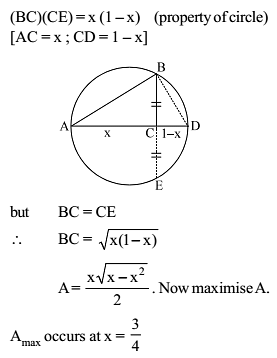PCM - Mock Test (30 Sep) - JEE MCQ
30 Questions MCQ Test Daily Test for JEE Preparation - PCM - Mock Test (30 Sep)
A ideal gas is undergoing following thermodynamic cycle, consisting of isobaric, isothermal and adiabatic. The corresponding PT curve is : (approx.)

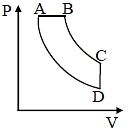
In pressure-volume diagram given below, the isochoric, isothermal, and isobaric parts respectively, are-

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
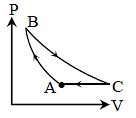
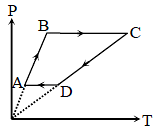
Six moles of an ideal gas performs a cycle shown in figure. If the temperature are TA = 600K,
TB = 800 K, TC = 2200K and TD = 1200 K, the work done per cycle is -

In an adiabatic process gas is reduced to quarter of its volume. What would happen to its pressure? Given ratio of specific heats γ= 2
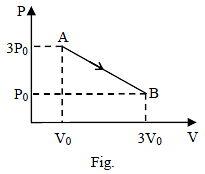
n moles of an ideal gas undergoes a process A to B as shown. Maximum temperature of gas during the process is –
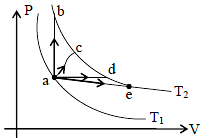
The figure shows two isotherms at temperatures T1 and T2. A gas is taken from one isotherm to another isotherm through different processes. Then change in internal energy ΔU has relation -
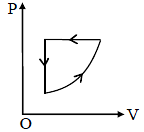
For one complete cycle of a thermodynamic process on a gas as shown in the P-V diagram. Which of the following is correct ?
In an adiabatic expansion of air the volume increases by 5%. What is the percentage change in pressure ? [(1.05)7/5 = 1.07]
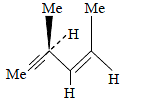
Hydrogenation of the above compound in the presence of poisoned pallodium catalyst gives
What would be the product when ethene is oxidised with cold dil. KMnO4 solution -
For an ideal solution of two components  and B, which of the following is true?
and B, which of the following is true?
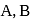 and
and  are in the order
are in the order 
 and 1 atmosphere partial pressure of hydrogen,
and 1 atmosphere partial pressure of hydrogen,  of hydrogen measured at STP dissolves in
of hydrogen measured at STP dissolves in  of water. If water at
of water. If water at  is exposed to a gaseous mixture having a total pressure of
is exposed to a gaseous mixture having a total pressure of  of
of  (excluding the vapour pressure of water) and containing
(excluding the vapour pressure of water) and containing  hydrogen by volumne, then the volume of hydrogen measured at STP that will dissolve in 1 L of water is
hydrogen by volumne, then the volume of hydrogen measured at STP that will dissolve in 1 L of water is

Hydrogenation of the above compound in the presence of poisoned pallodium catalyst gives
The number of structural and configurational isomers of a bromo compound, C5H9Br formed by the addition of HBr to 2-pentyne respectively -
Which of the following functions defined below are NOT differentiable at the indicated point?
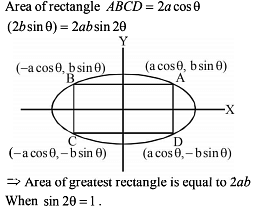
A right triangle is drawn in a semicircle of radius 1/2 with one of its legs along the diameter. The maximum area of the triangle is
|
360 tests
|


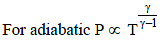
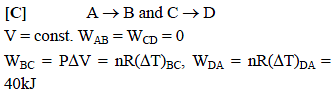
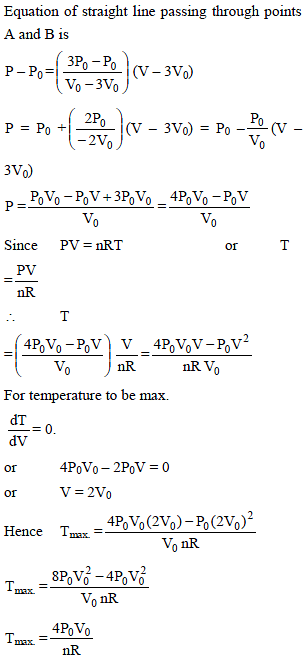
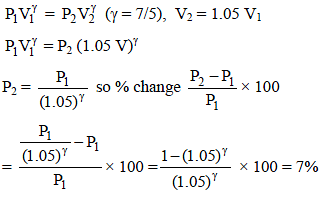
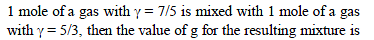
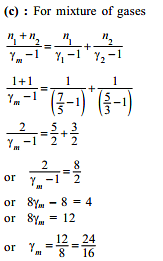







 i.e. as the solubility increases, value of Henry's law constant decreases. Since
i.e. as the solubility increases, value of Henry's law constant decreases. Since  is most soluble in water among the given set of gases. Therefore
is most soluble in water among the given set of gases. Therefore  has the lowest value of Henry's law constant.
has the lowest value of Henry's law constant.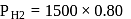
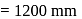 of
of 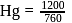 atmosphere
atmosphere 
 ; or
; or