Physics: Topic-wise Test- 6 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series 2025 - Physics: Topic-wise Test- 6
If radiation of allow wavelengths from ultraviolet to infrared is passed through hydrogen agas at room temperature, absorption lines will be observed in the :
In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed to 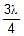 the kinetic energy of the fastest emitted electron will be :
the kinetic energy of the fastest emitted electron will be :
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
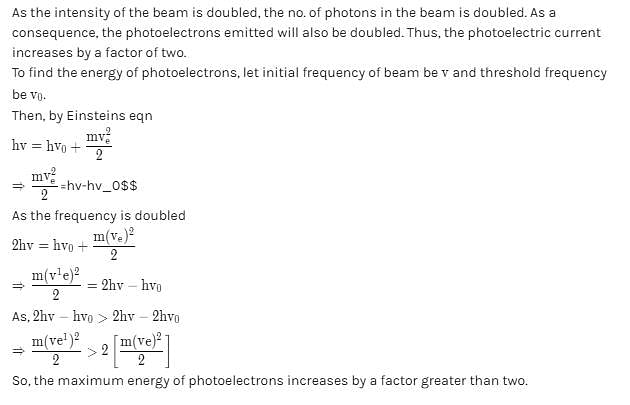
The frequency and the intensity of a beam of light falling on the surface of photoelectric material are increased by a factor of two (Treating efficiency of photoelectron generation as constant). This will :
Light coming from a discharge tube filled with hydrogen falls on the cathode of the photoelectric cell. The work function of the surface of cathode is 4eV. Which one of the following values of the anode voltage (in Volts) with respect to the cathode will likely to make the photo current zero.
In a photoelectric experiment, the potential difference V that must be maintained between the illuminated surface and the collector so as just to prevent any electron from reaching the collector is determined for different frequencies f of the incident illumination. The graph obtained is shown. The maximum kinetic energy of the electrons emitted at frequency f1 is
In photoelectric effect, stopping potential depends on
Two electrons are moving with the same speed v. One electron enters a region of uniform electric field while the other enters a region of uniform magnetic field, then after sometime if the de-Broglie wavelengths of the two are l1 and l2 then :
An electron in hydrogen atom first jumps from second excited state to first excited state and then, from first excited state to ground state. Let the ratio of wavelength, momentum and energy of photons in the two cases by x, y and z, then select the wrong answers :
An electron is in an excited state in hydrogen-like atom. It has a total energy of –3.4 eV. If the kinetic energy of the electron is E and its de-Broglie wavelength is l, then
A particular hydrogen like atom has its ground state binding "energy 122.4 eV. Its is in ground state. Then :
The electron in a hydrogen atom makes a transition n1 ¾→ n2, where n1 & n2 are the principal quantum numbers of the two states. Assume the Bohr model to be valid. The time period of the electron in the initial state is eight times that in the final state. The possible values of n1 & n2 are :
In hydrogen atom the kinetic energy of electron in an orbit of radius r is given by
According to Bohr model of hydrogen atom, the radius of stationary orbit characterized by the principal quantum number n is proportional to
Select an incorrect alternative:
i. the radius of the nth orbit is proprtional to n2
ii. the total energy of the electron in the nth orbit is inversely proportional to n
iii. the angular momentum of the electron in nth orbit is an integral multiple of h/2π
iv. the magnitude of potential energy of the electron in any orbit is greater than its kinetic energy
Which of the following is/are deduced from the Rutherford’s scattering experiment?
(1) There are neutrons inside the nucleus.
(2) The sign of the charge of the nuclei is the same as the sign of alpha particles.
(3) Electrons are embedded in the nucleus.
In scattering, the impact parameter b is defined as the:
The alpha particle scattering experiment was carried out by:
We know that the Rutherford model of the atom is superior to the Thompson model because when alpha particles are scattered from atoms:
In Rutherford’s experiment, a thin gold foil was bombarded with alpha particles. According to Thomson’s “plum-pudding” model of the atom, what should have happened?
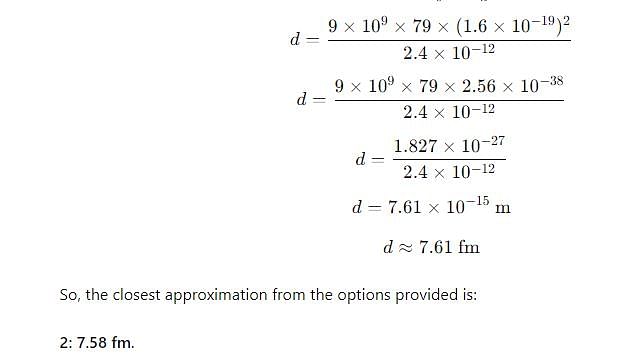
The distance of closest approach when a 15.0 MeV proton approaches gold nucleus (Z = 79) is
The targets used in the alpha particle atomic experiments in the early 1900’s was:
What percentage of the mass of an atom is concentrated in the nucleus?
Plutonium decays with a half-life of 24000 years. If the plutonium is stored for 72000 years, then the fraction of plutonium that remains is
In a nuclear reaction which of the following is conserved
If 10% of a substance decays in 10 days, then approximate percentage of substance left after 24 days is
Choose the WRONG statement. A thermonuclear fusion reactor is better than a fission reactor for the following reason:
Which of the following is true for a forward-biased diode?
In an unbiased p-n junction, holes diffuse from the p-region to n-region because
|
1 videos|26 docs|111 tests
|
|
1 videos|26 docs|111 tests
|


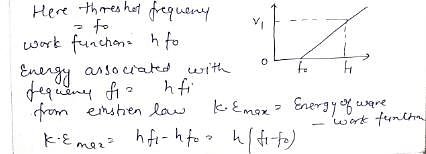
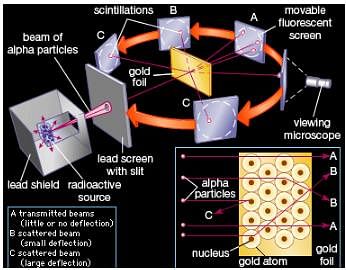 Physicist Ernest Rutherford established the nuclear theory of the atom with his gold-foil experiment. When he shot a beam of alpha particles at a sheet of gold foil, a few of the particles were deflected. He concluded that a tiny, dense nucleus was causing the deflections.
Physicist Ernest Rutherford established the nuclear theory of the atom with his gold-foil experiment. When he shot a beam of alpha particles at a sheet of gold foil, a few of the particles were deflected. He concluded that a tiny, dense nucleus was causing the deflections.














