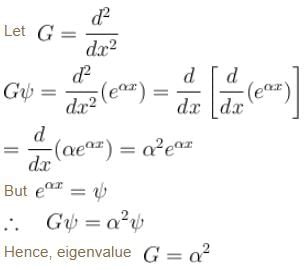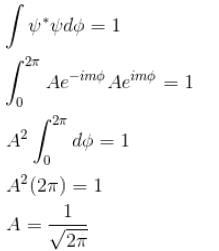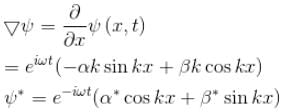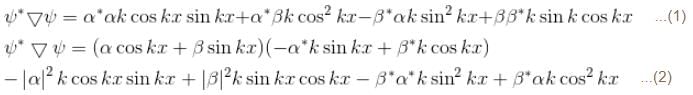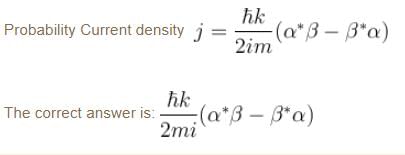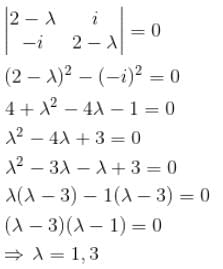Postulates Of Quantum Mechanics MCQ Level - 1 - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - Postulates Of Quantum Mechanics MCQ Level - 1
A certain excited state of hydrogen atom is known to have a life of 2.5 × 10–14 s. The minimum error, with which the energy of the excited state can be measured is.
Which of the following is the eigenfunction of the linear momentum operator 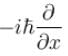 with a positive eigenvalue
with a positive eigenvalue 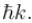
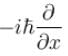 with a positive eigenvalue
with a positive eigenvalue 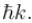
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
An eigenfunction of an operator 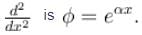 The corresponding eigenvalue is.
The corresponding eigenvalue is.
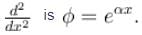 The corresponding eigenvalue is.
The corresponding eigenvalue is.The ground state energy of an electron confined to a box 1Å wide is.
Which of the following values of A will properly normalize the wave function of a rigid dumbbell rotating about its centre having φ dependence as ψ(φ) = Aeimφ where m is a quantum number.
A particle of mass m has the wave function ψ(x, t) = eiωt (α cos kx + β sin kx) where α, β are complex constants and ω and k are real constants. The probability current density is equal to :
If de-Broglie wavelength of an electron is 7.3Å. Then the velocity of electron is. Given, me = 9.1 × 10–31 kg and h = 6.6 × 10–34 Js.
Which of the following is correct set of eigenvalues of the Hermitian matrix 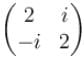
If ψ is a normalized solution of the Schrödinger equation and Q is the operator corresponding to a physical quantity x, then the quantity 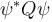 may be integrated in order to obtain :
may be integrated in order to obtain :
A particle limited to the x–axis has the wave frequency ψ = ax between x = 0 and x = 1, ψ = 0 elsewhere. The expectation value 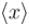 of the particle's position is.
of the particle's position is.


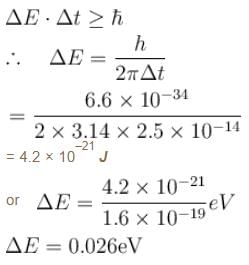
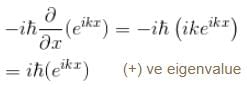
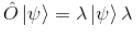 is the eigenvalue
is the eigenvalue