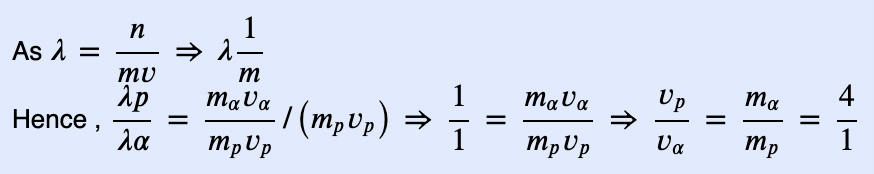QUIZ 2:Photoelectric Effect(#freetestseries) - JEE MCQ
15 Questions MCQ Test - QUIZ 2:Photoelectric Effect(#freetestseries)
The idea of matter waves was given by
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The de-Broglie wavelength associated with the particle of mass m moving with velocity v is [CBSE PMT 1992]
A photon, an electron and a uranium nucleus all have the same wavelength. The one with the most energy [MP PMT 1992]
When the kinetic energy of an electron is increased, the wavelength of the associated wave will
If the de-Broglie wavelengths for a proton and for a α−particle are equal, then the ratio of their velocities will be [NCERT 1972]
A particle which has zero rest mass and non-zero energy and momentum must travel with a speed [MP PMT 1992; DPMT 2001; Kerala PMT 2004]
Dual nature of radiation is shown combinedly by [MP PET 1991]
For the Bohr's first orbit of circumference 2πr, the de-Broglie wavelength of revolving electron will be [MP PMT 1987]
An electron of mass m when accelerated through a potential difference V has de-Broglie wavelength λ. The de-Broglie wavelength associated with a proton of mass M accelerated through the same potential difference will be [CBSE PMT 1995; EAMCET 2001; J & K CET 2004]
What will be the ratio of de-Broglie wavelengths of proton and α−particle of same energy [RPET 1991, 96; DCE 2002; Kerala PET 2005]
What is the de-Broglie wavelength of the α−particle accelerated through a potential difference V [RPMT 1996]
The energy that should be added to an electron, to reduce its de-Broglie wavelengths from 10-10 m to 0.5×10-10m, will be [KCET (Engg./Med.) 2000]
If particles are moving with same velocity, then maximum de-Broglie wavelength will be for [CBSE PMT 2002]
If an electron and a photon propagate in the form of waves having the same wavelength, it implies that they have the same [CBSE PMT 1995; DCE 2001; AIIMS 2003]