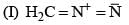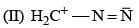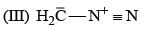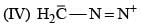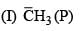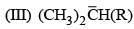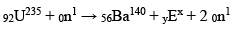Quanta Test - Chemistry MCQ
30 Questions MCQ Test - Quanta Test
Relative lowering of vapour presusure produced by dissolving 71.5g of a substance in 1Kg of wa0.ter is 0.0073. Molecular weight of substance will be
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The relationship been Osmotic pressure at 273K when 10g Glucose (P1), 10g Urea (P2), 10g Sucrose (P3) are dissolved in 250 Ml of water (P3) are dissolved in 250 Ml of water
Solution A contains 7g/L of MgCl2 and Solution B contains 7g/L of NaCl. At room temp, Osmotic pressure of
When a substance is dissolved in a solvent the vapour pressure of the solvent is decreased. This results in
At a given temperature, total vapour pressure in Torr of a mixture of volatile components A and B is given by p = 120 – 75XB Hence, vapour pressure of pure A and B respectively (in Torr) are:
If the ΔG of a cell reaction AgCl– + e– → Ag + Cl– is –21.20 KJ; the standard e.m.f., of cell is:
Relative stabilities of the following carbocation will be in the order:
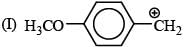
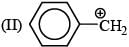
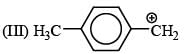

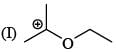
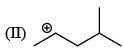
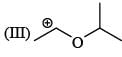
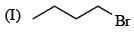
There is formation of precipitate almost instantly when a alcoholic AgNO3 reacts with
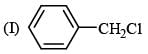
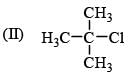
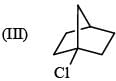
Leaving tendency of the following in increasing order is:
(I) Cl–
(II) CH3COO–
(III) OH–
(V) RO–
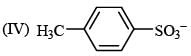
(VI) NH3–
E1, E2 and E3 are the emf values of the three galvanic cells respectively.


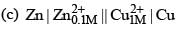
For the electrochemical cell,
M|M+|| X–| X, E° (M+/M) = 0.44 V E°(X/X–) = 0.33 V. From this data one can deduce that:
Based on the following information,
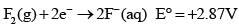
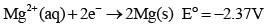
Which of the following chemical species is the strongest reducing agent?
The standard reduction potentials at 298 K for the following half reactions are given against each
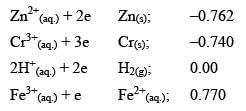
Which is the strongest reducing agent
Most radioactive of the isotopes of an element is the one with largest value of
T1/2 of C14 isotope is 5770 years. Time after which 72% of isotope left is:
2 g of a radioactive sample having half life of 15 days was synthesized on 1st Jan 2018. The amount of the sample left behind on 1st March, 2018 (including both the days)
In the sequence of following nuclear reactions

The value of n will be
58.5 g of NaCl and 180g of glucose were separately dissolved in 1L of water. Identify the correct statements regarding the elevation in boiling point of solution.
Elevation in boiling point of solution of 13.44g of CuCl2 in 1 Kg of water will be (Mwt = 134.4, Kb = 0.52Kmolal–1, assume it is a strong electrolyte)


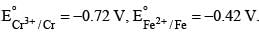 The potential for the cell
The potential for the cell 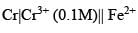 (0.01 M)|Fe is:
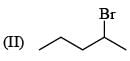
(0.01 M)|Fe is: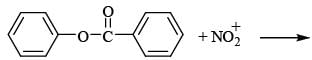 Product of this reaction by single SE reaction:
Product of this reaction by single SE reaction: