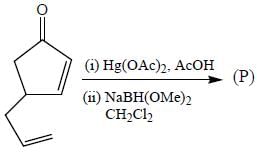Reactive Inter & Reaction Mechanism - GATE Chemistry MCQ
20 Questions MCQ Test GATE Chemistry Mock Test Series - Reactive Inter & Reaction Mechanism
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
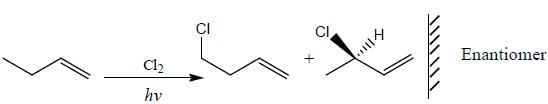
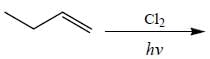
How many monochlorinated products are possible including stereoisomer?
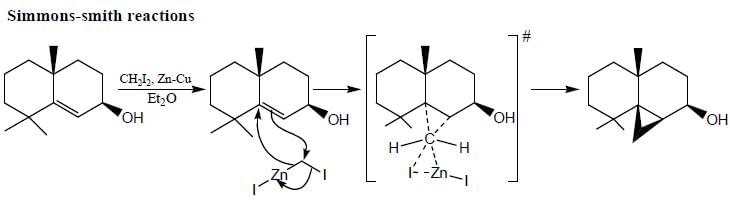
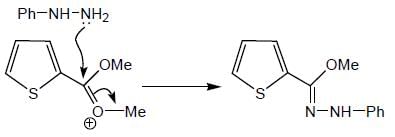
Product formed in the above reaction, which of the following correct mechanism apply
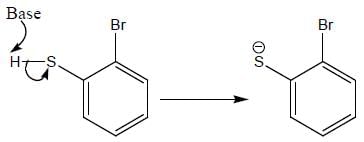
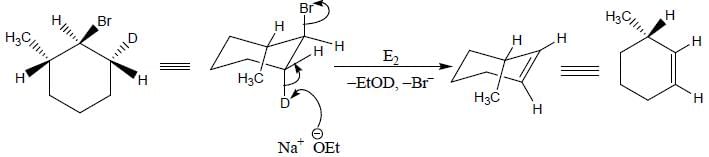
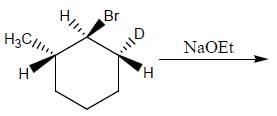
The reaction of the bromo compound shown below with sodium ethoxide gives predominantly
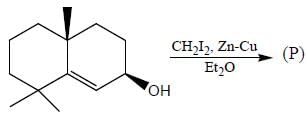
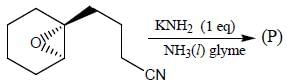
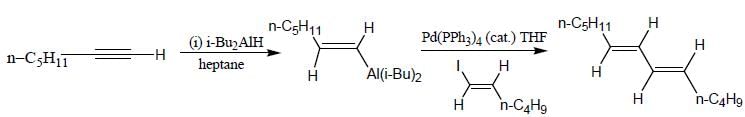
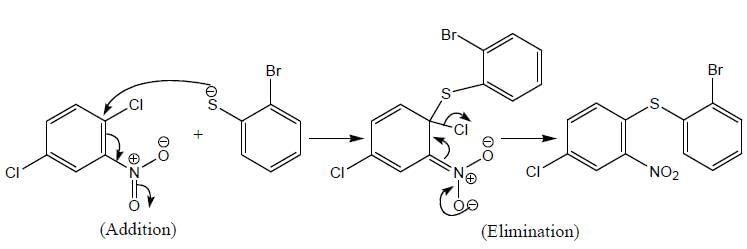
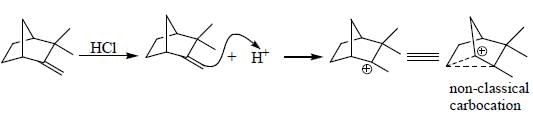
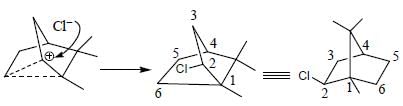
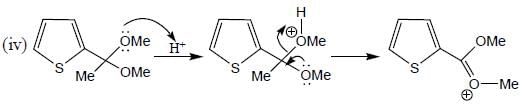
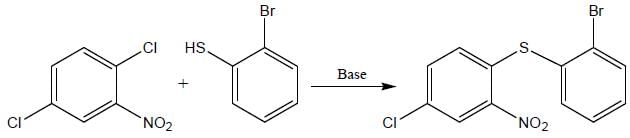
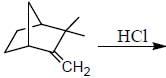
The major product obtained in the reaction below is
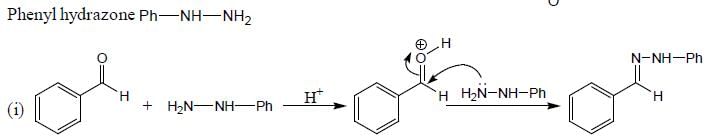
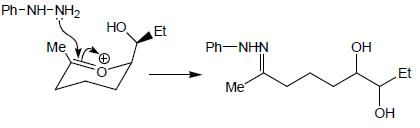
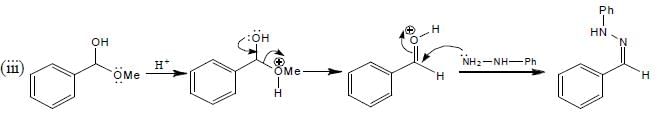
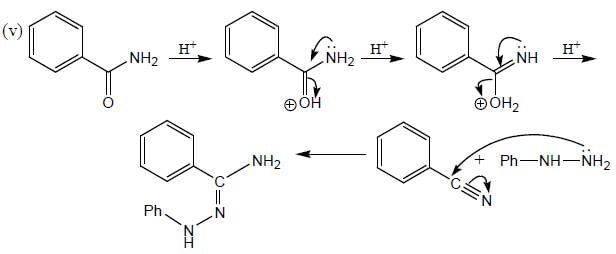
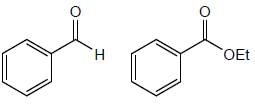
The total number of compounds (shown below) that form phenylhydrazone derivatives under acidic conditions is ____________
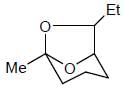
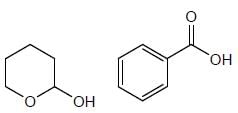
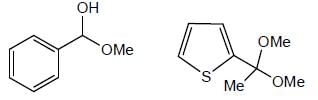
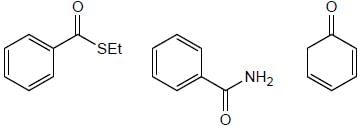
How many product will be formed the following reaction is
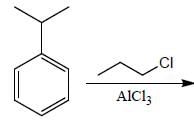
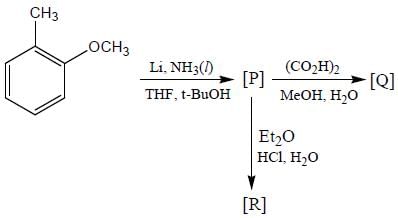
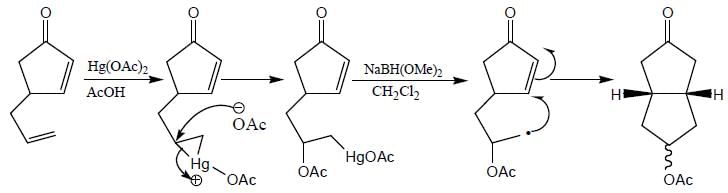
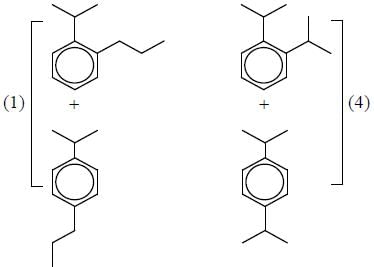
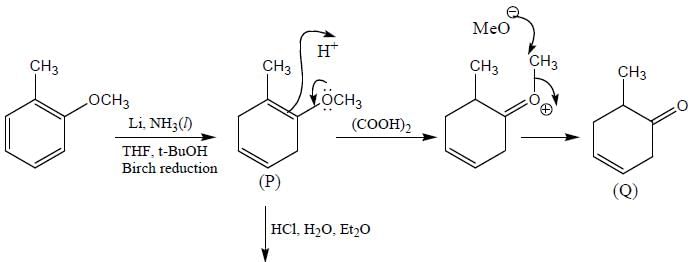
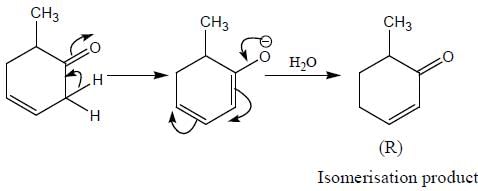
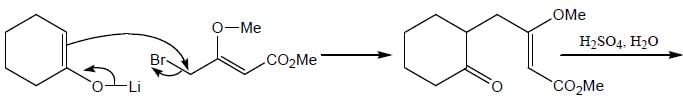
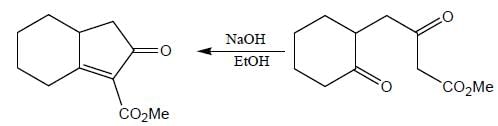
Correct product (Q, R and P) in the above reactions are formed respectively
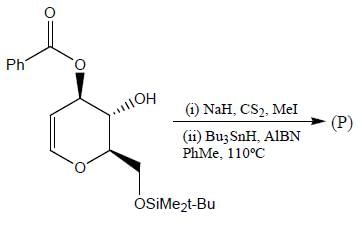
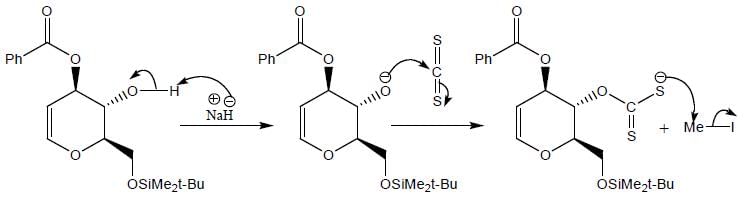
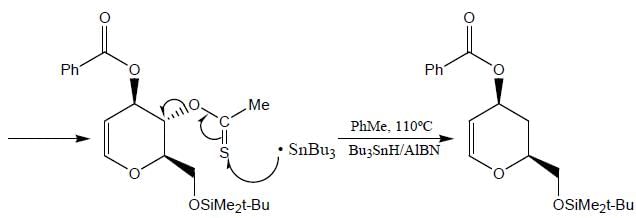
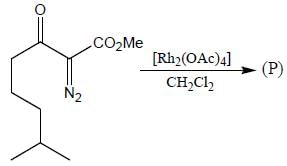
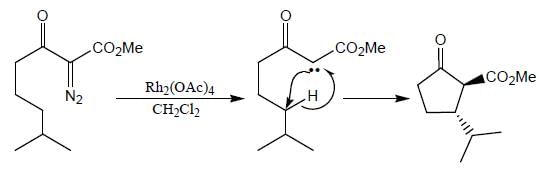
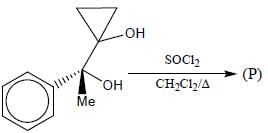
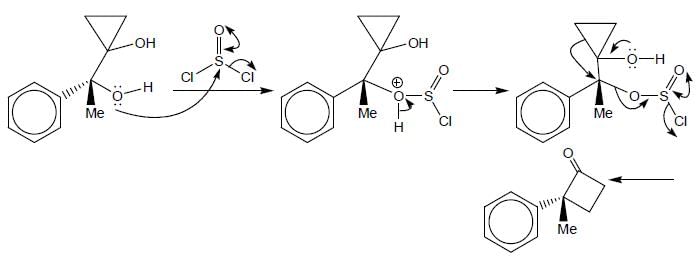
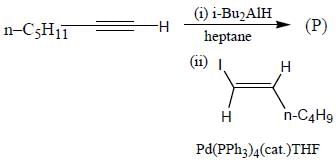
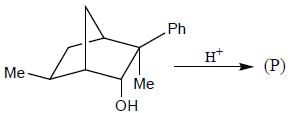
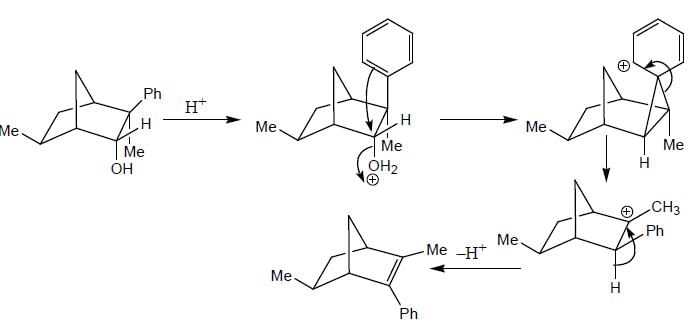
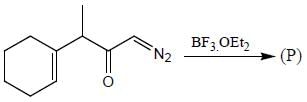
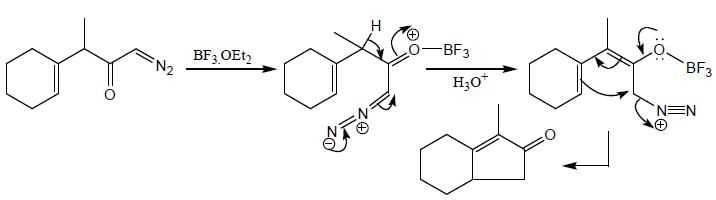
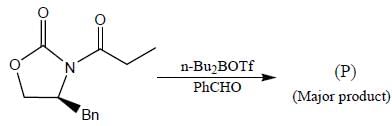
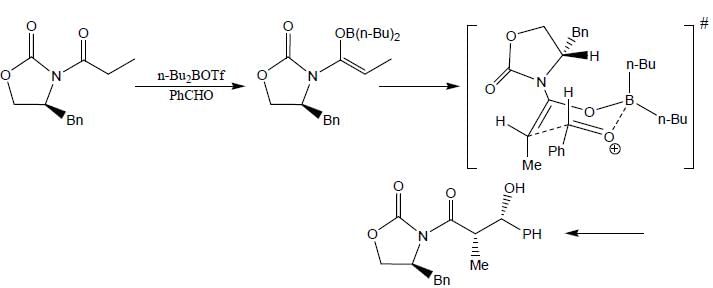
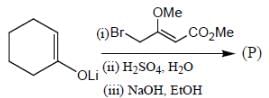
The major product (P) in the above reaction is
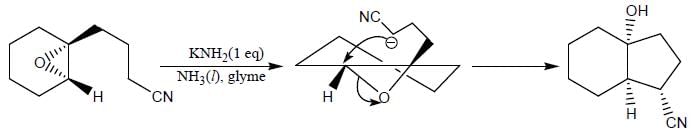
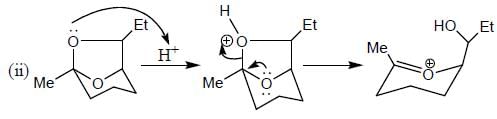
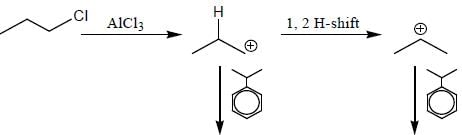
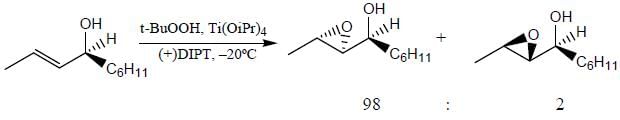
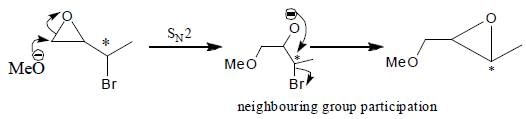
Which of the following statement(s) is true about the reaction given below?
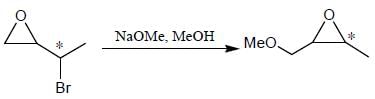
(1) it involves a carbocation intermediate
(2) rearrangement is due to SN1 reaction mechanism.
(3) it proceeds via a concerted SN2 pathway
(4) it involves neighbouring group participation.
|
18 docs|37 tests
|
|
18 docs|37 tests
|