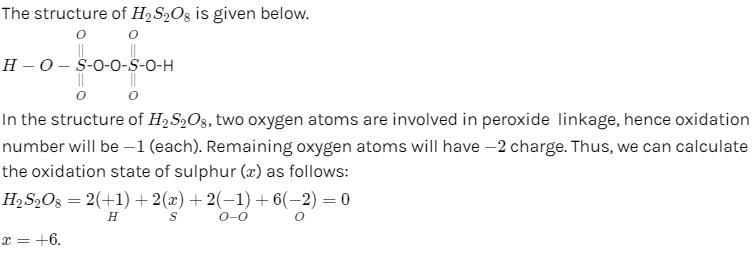Redox Reactions (Chapter Test - Medical) - Class 12 MCQ
20 Questions MCQ Test - Redox Reactions (Chapter Test - Medical)
Oxidation states of carbon atoms in diamond and graphite are
Which of the following has been arranged in order of increasing oxidation number of nitrogen ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In which of the following reactions, the underlined substance is oxidised ?
Which of the following statements is correct ?
The reaction of calcium with water is represented by the equation
What volume of H2 at STP would be liberated when 8 g of calcium completely reacts with water ?
In the balanced chemical reaction a, b, c and d respectively correspond to
In which of the following reaction H2O2 does not act as oxidising agent ?
The oxidation number of phosphorous in Mg2P2O7 is
For reducing one mole of Fe2+ ion to Fe, the number of faraday of electricity is
If HNO3 changes to N2O, the oxidation number is changed by
Which of the following have been arranged in order of decreasing oxidation number of sulphur ?
Given : Which of the following fact is correct ?
Adding powdered Pb and Fe to a solution containing 1M in each of Pb2+ and Fe2+ ions would result into the formation of
A gas Y of 1 atm is bubbled through a solution containing a mixture of 1 MX and 1 MZ at 25°C. If the reduction potential of Z > Y > X, then
Which of the following will act as cathode when connected to standard hydrogen electrode which has E0 value given as zero ?
Which of the following is not a correct statement about electrochemical series of reduction potentials?