Retro (Past 13 Year) IIT-JEE Advanced (Aliphatic Aldehydes And Ketones) - JEE MCQ
18 Questions MCQ Test - Retro (Past 13 Year) IIT-JEE Advanced (Aliphatic Aldehydes And Ketones)
The major product of the following reaction is
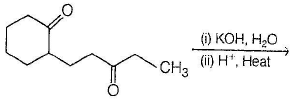
The major product in the following reaction is
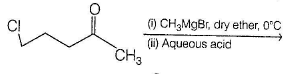
(2014 Adv., Only One Option Correct Type)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Consider all possible isomeric ketones including stereoisomers of MW = 100. All these isomers are independently reacted with NaBH4. The total number of ketones that give a racemic product(s) is/are
Note Stereoisomers are also reacted separately.
(2014 Adv., Integer Type)
The major product H in the given reaction sequence is
(2012, Only One Option Correct Type)
The number of aldol reaction (s) that occurs in the given transformation is
(2012, Only One Option Correct Type)
In the scheme given below, the total number of intramolecular aldol condensation products formed from 'Y' is
Passage for Q. Nos. (7-9)
Two aliphatic aldehydes P and Q react in the presence of aqueous K2CO3 to give compound R, which upon treatment with HCN provides compounds. On acidification and heating, S gives the product shown below :
Q.
The compounds P and Q respectively are
(2010, Comprehension Type)
Two aliphatic aldehydes P and Q react in the presence of aqueous K2CO3 to give compound R, which upon treatment with HCN provides compounds. On acidification and heating, S gives the product shown below :
Q.
The compound R is
Two aliphatic aldehydes P and Q react in the presence of aqueous K2CO3 to give compound R, which upon treatment with HCN provides compounds. On acidification and heating, S gives the product shown below :
Q.
The compound S is
Passage for Q. Nos. (10-12)
A carbonyl compound P, which gives positive iodoform test, undergoes reaction with MeMgBr followed by dehydration to give an olefin Q. Ozonolysis of Q leads to a dicarbonyi compound R, which undergoes intramolecular aldo reaction to give predominantly S.
Q.
The structure of the carbonyl compound P, is
A carbonyl compound P, which gives positive iodoform test, undergoes reaction with MeMgBr followed by dehydration to give an olefin Q. Ozonolysis of Q leads to a dicarbonyi compound R, which undergoes intramolecular aldo reaction to give predominantly S.
Q.
The structure of the product S, is
A carbonyl compound P, which gives positive iodoform test, undergoes reaction with MeMgBr followed by dehydration to give an olefin Q. Ozonolysis of Q leads to a dicarbonyi compound R, which undergoes intramolecular aldo reaction to give predominantly S.
Q.
The structures of the products Q and R, respectively, are
Passage for Q. Nos. (13-15)
In the following sequence, product I, J and L are formed , K represents a reagent.
Q.
The structure of the product I is
(2008, Comprehension Type)
In the following sequence, product I, J and L are formed , K represents a reagent.
Q.
The structures of compounds J and K, respectively, are
In the following sequence, product I, J and L are formed , K represents a reagent.
Q.
The structure of product L is
Cyclohexene on ozonolysis followed by reaction with zinc dust and water gives compound E. Compound E on further treatment with aqueous KOH yields compound F. Compound F is
(2007, Only One Option Correct Type)
The smallest ketone and its next homologue are reacted with NH2OH to form oxime
(2006, Only One Option Correct Type)
Butan-2-one can be converted to propanoic acid by which of the following ?
(2006, Only One Option Correct Type)

















