Revisal Problems (Past 13 Year) JEE Advanced (Alcohols Phenols And Ethers) - JEE MCQ
30 Questions MCQ Test - Revisal Problems (Past 13 Year) JEE Advanced (Alcohols Phenols And Ethers)
Only One Option Correct Type
Direction (Q. Nos. 1-12) This section contains 12 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Consider the following reaction,
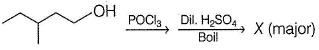
Q.
The correct statement regarding X is
Reaction of (+) - 3 - methylcyclopentene with (i) Cl2, H2O and (ii) NaH will provide
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
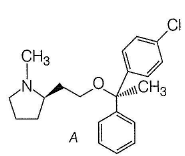
From which alcohol and which alkyl halide could A be best prepared through Williamson ether synthesis?
A synthesis of 2,5 - dimethyl- 2 -hexanol from 2 - methylpropene requires the for mation of two four carbon intermediates, X and Y. These intermediates combine to give the desired product after the usual hydrolysis work-up. Select the appropriate methods of preparing X and Y from 2-methylpropene.
What is the major product of the following reaction?
A chiral C5H10O alcohol is reduced by catalytic hydrogenation to an achiral C5H12O alcohol. The original alcohol is oxidised by activated MnO2 to an achiral carbonyl compound (C5H8O). Which of the following might be the chiral alcohol?
The Lucas test is used to distinguish small (7 or fewer carbons) 1°, 2° and 3° alcohols. The alcohol to be tested is added to a solution of anhydrous ZnCI2 in conc. HCI at room temperature. Which of the following statements is not correct ?
If the following 13C labelled compound undergo the following reaction, which product would be obtained ?
Which sets of reagents shown below would accomplish the fo llow ing transform ation?
A C5H12O compound is optically active, and is oxidised by PCC in CH2Cl2 to an optically active C5H10O product, which is racemised in acid or base. Which of the following best fits these facts ?
What is the major product of the reaction?
When treated with acid, the strained ether (I) is protonated on oxygen, and ring opens to give a resonance stabilised carbocation. Which diene listed below when treated with acid will give the same carbocation ?
One or More than One Options Correct Type
Direction (Q. Nos. 13-18) This section contains 6 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
The reaction producing 2-ethyl-2-methyl oxirane is/are
Compounds which on reduction can produce alcohols is/are
Lucas test is used for distinguishing primary, secondary.and tertiary alcohols as:
Q.
The correct statement regarding the above test is/are
3-methyl-3-hexano! can be prepared by the reaction of
The correct statement regarding the product(s) of the following reaction is/are
Which of the following Grignard synthesis would fail to produce the desired alcohols?
Comprehension Type Direction
(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage I
A and B are two structural isomers with their molecular formula C7H14O and both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or B with hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2O gives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.
Q.
Which of the following statement regarding A and B is correct?
Passage I
A and B are two structural isomers with their molecular formula C7H14O and both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or B with hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2O gives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.
Q.
Which of the following statement regarding A and B is incorrect?
Passage I
A and B are two structural isomers with their molecular formula C7H14O and both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or B with hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2O gives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.
Q.
Which of the following statement regarding acid catalysed isomerisation of A and B is true?
Passage II
An organic compound A(C9H10O) is chiral and does not evolve any gas on treatment with Na-metal but on hydrolysis with dil. H2SO4 gives B (C9H12O2) which on further treatment with alkaline solution of iodine does not give an yellow precipitate. Also A on treatment with excess of conc. HBr gives C (C9H11OBr) as the major product. C on further treatment with C2H5ONa/C2H5OH followed by acidification of product gives D (an isomer of A). D on ozonolysis followed by work-up with dimethyl sulphide gives E (C8H8O2) as one of the product.
Q.
The most likely structure of starting compound A is
Passage II
An organic compound A(C9H10O) is chiral and does not evolve any gas on treatment with Na-metal but on hydrolysis with dil. H2SO4 gives B (C9H12O2) which on further treatment with alkaline solution of iodine does not give an yellow precipitate. Also A on treatment with excess of conc. HBr gives C (C9H11OBr) as the major product. C on further treatment with C2H5ONa/C2H5OH followed by acidification of product gives D (an isomer of A). D on ozonolysis followed by work-up with dimethyl sulphide gives E (C8H8O2) as one of the product.
Q.
Which of the following statement regarding B is correct?
Passage II
An organic compound A(C9H10O) is chiral and does not evolve any gas on treatment with Na-metal but on hydrolysis with dil. H2SO4 gives B (C9H12O2) which on further treatment with alkaline solution of iodine does not give an yellow precipitate. Also A on treatment with excess of conc. HBr gives C (C9H11OBr) as the major product. C on further treatment with C2H5ONa/C2H5OH followed by acidification of product gives D (an isomer of A). D on ozonolysis followed by work-up with dimethyl sulphide gives E (C8H8O2) as one of the product.
Q.
The statement that is true regarding A to D is
Matching List Type
Direction(Q. Nos. 25-27) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q.
Match the Column I with Column II and mark the correct option from the codes given below.
Match the Column I with Column II and mark the correct option from the codes given below.
Match the Column I with Column II and mark the correct option from the codes given below.
One Integer Value Correct Type
Direction (Q. Nos. 28-30) This section contains 3 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
An ether, on treatment with sulphuric acid and sodium iodide, yields a single iodoalkane that contains 74.7% iodine by weight. How many carbon atoms are present in a molecule of this ether ? [Molar mass of I = 127]
An organic compound A (C10H18O8)on treatment with excess of CH3COCI gives a fully acetylated product whose molar mass is found to be 518 g/mol. How many hydroxyl functional groups are present in A?
How many different triols would be formed when the following compound is treated with excess of CH3MgBr followed by hydrolysis ?

















