Revisal Problems (Past 13 Year) JEE Advanced (Aliphatic Aldehydes And Ketones) - JEE MCQ
30 Questions MCQ Test - Revisal Problems (Past 13 Year) JEE Advanced (Aliphatic Aldehydes And Ketones)
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
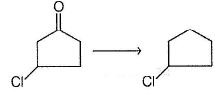
The best reagent that can bring about the following conversion is
Which carbon-carbon bond could not be formed by an aldol condensation reaction?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Arrange the following in the increasing order of hydrate content when dissolved in water.
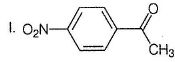
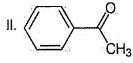
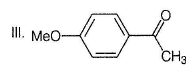
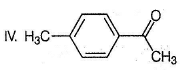
What is the major product in the following sequence of reaction?
An organic compound A (C6H12O) neither decolourise bromine water nor changes the colour of acidic dichromate solution. A on heaing with H2SO4 produces an alkene which on oxidative ozonolysis gives B (C6H10O3) which gives a yellow precipitate with NaOH/I2. The most probable structure of A is
Which of the following is not true regarding the reaction given below.
Which of the following compounds exchanges the largest number of hydrogens from deuterium when treated with KOD in D2O ?
Treatment of cyclohexanone with an excess of produced 18O labelled cyclohexanone. Which of the following is a likely intermediate in this isotope exchange? (the isotope is not named)
One or More than One Options Correct Type
Direction (Q. Nos. 9-14) This section contains 6 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Which is/are the expected products in the following reaction ?
Treatment of A (C5H12O) with concentrated sulphuric acid results in the formation of three alkenes in differing yields. Also, A forms a yellow precipitate on treatment with NaOH/I2. A turns colour of acidified dichromate solution to blue green, forming a new organic compound B which also forms yellow precipitate on treatment with NaOH/I2. The most likely name of A is
The carbonyl compound(s) that will undergo racemisation on treatment with aqueous KOH is(are)
Which of the following reagants can bring about the transformation shown below ?
Which of the following reactions has/have equilibrium constant (Kc) greater than one?
Which of the following, on treatment with NaHSO3(aq), will produce mixture of salts which can be separated by fractional crystallisation?
Statement Type
Direction(Q. Nos. 15 and 16) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : When benzaldehyde (C6H5CHO) is treated with concentrated NaOD solution in D2O , Cannizaro reaction occur but no C—D (bonds with deuterium) is formed.
Statement II : Cannizaro reaction involves hydride transfer mechanism.
Statement I : Consider the reaction given below,
Statement II : Aldehydes and ketones react with semicarbazide to form semicarbazone.
Comprehension Type
Direction (Q. Nos. 17-22) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage I
Q.
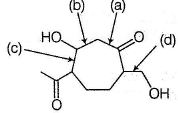
Which of the follwing can not be B ?
Passage I
Q.
Which of the following is most likely structure of C ?
Passage I
Q.
Which of the following most likely structure of D ?
Passage II
A chiral organic compound A (C9H12O2) does not give positive Tollen's test or iodoform test. However, A on treatment with 1.0 equivalent of CH3MgBr followed by acid hydrolysis gives B (C10H16O2) which gives positive iodoform test. B on refluxing with aqueous Na2CO3 solution gives the following compound as major product.
Q.
What is the most probable structure of A ?
Q.
B on treatment with hydrazine (N2H4) gives a cyclic hydrazone. What is the structure of hydrazone?
Passage II
A chiral organic compound A (C9H12O2) does not give positive Tollen's test or iodoform test. However, A on treatment with 1.0 equivalent of CH3MgBr followed by acid hydrolysis gives B (C10H16O2) which gives positive iodoform test. B on refluxing with aqueous Na2CO3 solution gives the following compound as major product.
Q.
If compound B is treated with excess of CH3MgBr followed by acid hydrolysis, how many different alcohols would be obtained?
Matching List Type
Direction (Q. Nos. 23-25) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q.
Match the Column I with Column II and mark the correct option from the codes given below.
Match the Column I with Column II and mark the correct option from the codes given below.
Match the following reactions of Column I with products from the Column II.
One Integer Value Correct Type
Direction (Q. Nos. 26-30) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
If propanal (CH3CH2CHO) is treated with DCI in D2O, keto-enol equilibria are established. At equilibrium, how many different enols would be existing?
Butanone + Dil. NaOH → Aldol (C8H16O2)
How many different aldol would be formed?
How many different alcohols would be formed in the above reaction?
In the reaction given below, how many different oximes would be formed?
Consider the following reactions,
Q.
How many different amides are formed in the above reactions?

















