Revisal Problems (Past 13 Year) JEE Main (Alcohols Phenols And Ethers) - JEE MCQ
30 Questions MCQ Test - Revisal Problems (Past 13 Year) JEE Main (Alcohols Phenols And Ethers)
Only One Option Correct Type
Direction (Q. Nos. 1-26) This section contains 26 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
What is the major product of the reaction?
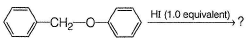

What is the major product of the reaction?
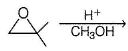
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What is the product of the reaction?
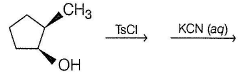
What is/are the possible product of the reaction?
What is the way to synthesise?
Which reaction will best perform the synthetic transform ation?
Which organic compound, will undergo the synthetic transformation ?
Which would be the best reagant to convent compound A to B ?
Which of the following diol(s) will not undergo a periodate (HIO4) cleavage?
What is the major product of the reaction?
Which of the following is not a reactive intermediate in the mechanism of the following reaction producing either product ?
Predict the major product of the reaction.
Which of the following alcohols cannot be synthesised using the reaction sequence ?
Which of the following pairs of the compounds can be used as starting materials in the synthesis of 2-phenyl-2-hexanol?
Which of the following sequence can be used to make
Which of the following reaction will most efficiently synthesise compound X (3-methyl-3-methoxy hexane) from 2-butanone, 1-chioropropane and methyl iodide ?
When 4-pentene-1 -ol is treated with aqueous bromide, a cyclic bromosubstituted ether is formed rather than expected bromohydrane. Select the explanation that the best account for the result.
Which of the following will react with periodic acid to produce an aldehyde product ?
Which reaction condition would be best to perform the following transformation?
What will be the major product of the reaction ?
What is the major product of the reaction?
Consider the molecule,
Q.
Which reagent will not give a positive test with this compound ?
Which reagent w ould provide the product shown ? 2-methyl oxirane
Chloroethane, C2H5CI, does not react with methanol under mild conditions. What reagent could be added to the reaction mixture to increase the rate of substitution?
Which reaction conditions would be best for the synthesis of isobutyl sec-butyl ether CH3CH2CH (CH3)-O-CH2CH(CH3)2?
A C6H14O chiral alcohol is converted to a bromide by treating with PBr3. Reaction of this bromide, first with Mg in ether, followed by quenching in 0.1 N HCI produces an achiral C6H14 hydrocarbon. Which of the following is the original alcohol ?
Direction (Q. Nos. 27-30) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : When 1-prop an ol is refluxed with dil. H2SO4, it isomerises to propanol.
Statement II : 2-propanol is more stable than 1-propan
Statement I : Diphenyl ether (Ph — O — Ph) is very less reactive in acid catalysed hydrolysis to phenol.
Statement II : Oxygen is in resonance on both side with phenyl ring.
Statement I : 3-methyl-2-butanol is more reactive than 2-butanol in acid catalysed dehydration to alkene.
Statement II : 3-methyl-2-butanol forms more stable carbocation than 2-butanol during dehydration reaction.
Statement I : Reaction of C2H5ONa with 2-chloro propane is a better method than the reaction of (CH3)2 CHONa with chloro ethane in order to prepare ethyl-isopropyl ether.
Statement II : Here ether is formed by SN2 reaction mechanism

















