Revisal Problems (Past 13 Years) JEE Advanced (Thermodynamics) - JEE MCQ
20 Questions MCQ Test - Revisal Problems (Past 13 Years) JEE Advanced (Thermodynamics)
Direction (Q. Nos. 1-7) This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Given,




Q. Thus, ΔfH° (HS) is
I and II are two isotherms, between p and V
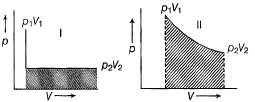
Select correct alternate
A 50.0 kg person drinks 500 g of milk which has a calorific value of approximately 720 cal g-1. If only 10% of the energy in milk is converted to mechanical work, how high (in meters) can the person climb based on this energy intake?
For the reaction,
Q. ΔG for this reaction at 298 K when the pressure of SO3 is 0.5 atm is
If the internal energy of a system decreases by 125 J and at the same time it absorbs 54 J of heat then
In compressing a gas, 355 J of work is done on the system. At the same time 185 J of heat escapes from the system. Thus, ΔE for the system is
0.4 mole of xenon is heated from 300 K to 400 K. Thus, change in internal energy is approximately (assume ideal behaviour and heat capacity is independent of temperature)
Direction (Q. No. 8) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : When liquid water freezes under constant pressure, work is done by the system.
Statement II : When liquid water freezes, volume increases.
Direction (Q. Nos. 9-11) This section contains 3 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. The standard enthalpies of combustion of fumaric acid and maleic acid are -1336.0 and -1359.2 kJ mol-1
ΔfH° (CO2) = - 393.5 kJ mol-1
ΔfH° (H2O) = - 285.8 kJ mol-1
Select correct alternates based on the following experiment.
A quantity of 0.850 mole of an ideal gas initially at a pressure 15.0 atm and 300 K is allowed to expand isothermally until its final pressure is 1.00 atm. Work done
A quantity of 200 mL of 0.862 M HCI is mixed with 200 mL of 0.431 M Ba(OH)2 in a constant pressure calorimeter that has a heat capacity of 453 JK-1. The initial temperature of the HCI and Ba(OH)2 solution is the same at 20.48°C. For the process the heat of neutralisation for
is -56.2 kJ mol-1.
Select correct statement(s)
Direction (Q. Nos. 12-17) This section contains 3 paragraphs each describing theory, experiments, data etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage I
The combustion of methane gas, the principal constituent of natural gas is represented by the equation
Q. Quantity of heat, in kilojoules, liberated in the complete combustion of 1.65 x 104 L of CH4(g) measured at 27°C and 760 mm Hg is
Passage I
The combustion of methane gas, the principal constituent of natural gas is represented by the equation
Q. Volume of water, in litres, that could be heated from 8.8°C to 68.8°C as a result of above heat is (density = 1 g/mL
Passage II
A system consisting of 1 mole of water vapour H2O(g) at 100°C and 1 atm with a volume of 30.12 L and condenses at a constant pressure of 1 atm to liquid water, H2O(l) at 100°C and 1 atm with a volume of 18.8 cm3.
ΔH = -40.7 kJ
Q. Work done in this transformation is
Passage II
A system consisting of 1 mole of water vapour H2O(g) at 100°C and 1 atm with a volume of 30.12 L and condenses at a constant pressure of 1 atm to liquid water, H2O(l) at 100°C and 1 atm with a volume of 18.8 cm3.
ΔH = -40.7 kJ
Q. ΔE of the process is
Passage III
Based on the following figure.
Q. ΔfH of NO2(g) is
Passage III
Based on the following figure.
Q. Heat of combustion of NO(g) is
Direction (Q. No. 18) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q. Acetic acid, CH3CO2H can form dimer (CH3CO2H)2 in the gas phase.
The dimer is held together by two hydrogen bonds with a total strength of 66.5 kJ mol-1 of dimer.
Direction (Q. Nos. 19 and 20) This section contains 2 questions. Each question, when worked out will result in integer from Oto 9 (both inclusive).
Q. A quantity of 0.50 mole of an ideal gas at 300 K expands isothermally against a constant pressure of 2.0 atm from 1.0 L to 5.0 L. Thus ΔS (universe) is .......
The molar heat capacity of oxygen at constant pressure is given by (25.7T + 0.01307) 10-3 kJ mol-1.
Calculate the enthalpy change (in kJ) when 1.45 moles of O2 are heated from 298 K to 367 K.














