S.A.-1 Pre Board - Online Test - Class 10 MCQ
30 Questions MCQ Test - S.A.-1 Pre Board - Online Test
The colour of the precipitate formed when barium chloride solution is mixed with sodium sulphate solution is :
A student heated small amount of ferrous sulphate in a test tube. She made the following observations.
(i) Ferrous colours sulphate changes to brown
(ii) A gas having a smell of burning sulphur is evolved
(iii) Water droplets collect on the upper side of the test tube.
(iv) Brown colured gas is evolved The correct set of observations is :
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A drop of a liquid sample was put on the pH paper. It was observed that the colour of the pH paper turned blue. The liquid sample is :
Two solutions X and Y were found to have pH value of 4 and 10 respectively. The inference that can be drawn is :
Which one of the following would you need to identify the gas that evolve when you heat NaOH solution with zinc metal ?
An iron nail was suspended in copper sulphate solution. After about one hour it was observed that the solution.
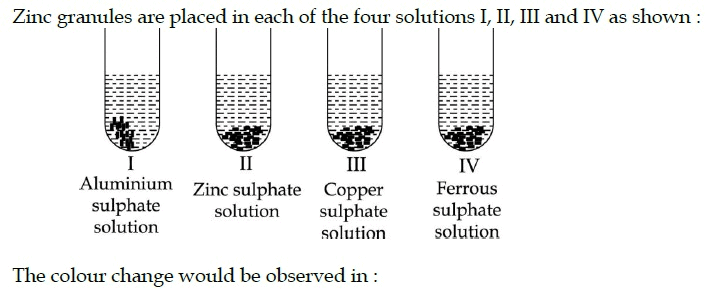
Freshly prepared aqueous solutions of ferrous sulphate and zinc sulphate respectively appear :
A student performed an experiment "To study the dependence of potential difference (V) across a resistor on current (I) flowing through it" by using four cells, each of 1.5 V. She connected the cells as shown below. The correct combination of cells to obtain 6V potential difference across XY is :
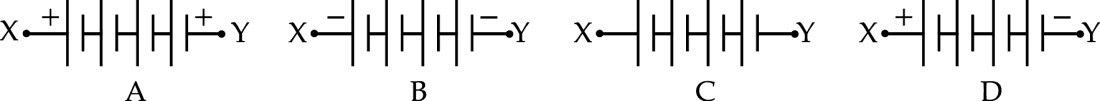
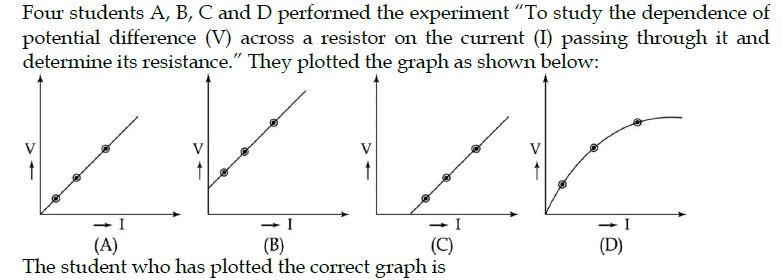
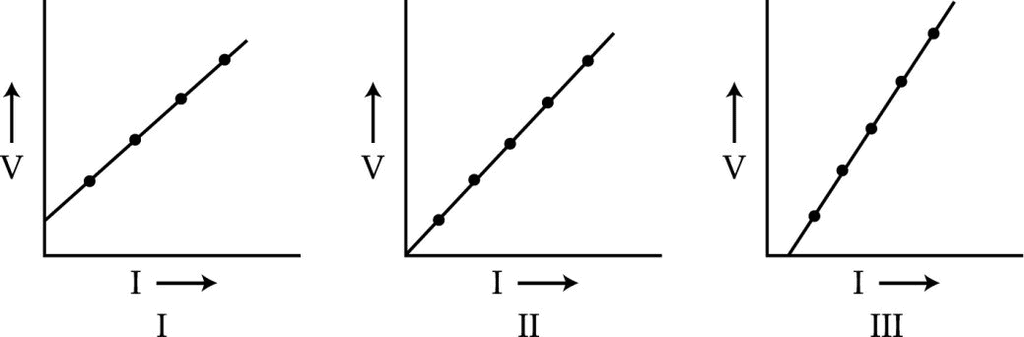
In the experiment „To study the dependence of potential difference on current (I), three students plotted the following graphs between V and I as per their respective observations.
The observations, likely to be correct are those of
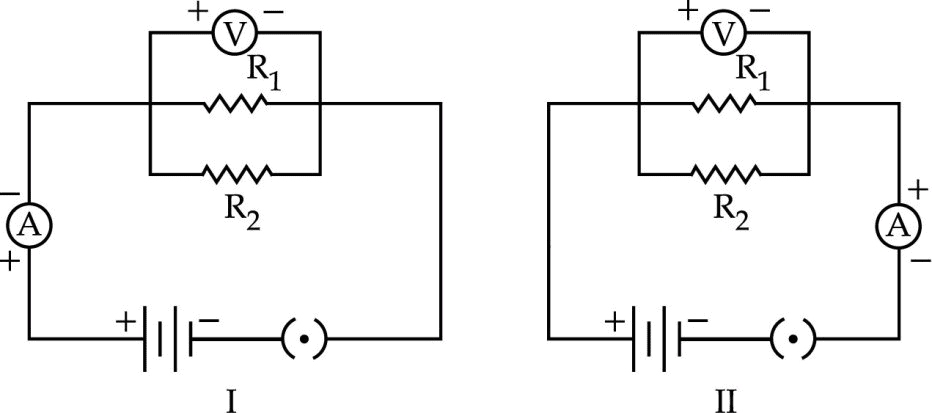
In the experiment „To find the equivalent resistance of two resistors, connected in parallel? two students connected the ammeter in two different ways as shown in the given circuits I and II. The ammeter has been correctly connected in :
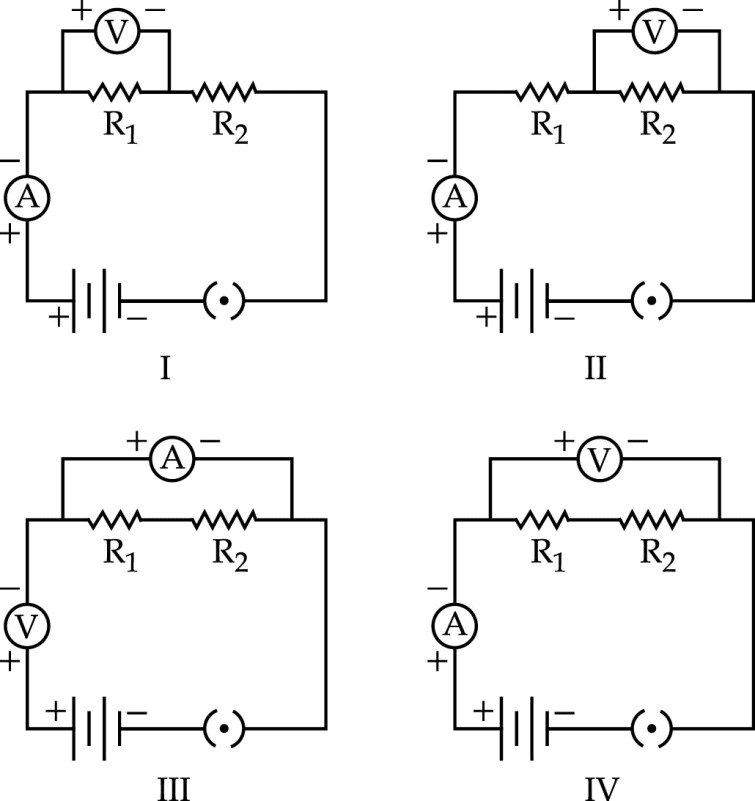
In the experiment on finding the equivalent resistance of two resistors, connected in series, the voltmeter across the combination is connected correctly only in circuit.
A student performed the experiment „To show that light is necessary for photosynthesis?. Before carrying out the test for the presence of starch in a leaf on exposure to sunlight, the leaf is put into alcohol contained in beaker and boiled over a water bath. This step is carried out to :
A student covered a portion of the experimental leaf from a destarched plant with a black paper strip and kept it in the garden outside. In the evening she tested the covered portion of the leaf for the presence of starch. The student was performing the experiment to verify that :
In order to prepare a temporary mount of a leaf peel for observing stomata, the chemicals used for staining and mounting respectively are :
Which of the following precautions should be kept in mind while preparing a temporary mount of an epidermal peel of a leaf :
I Wash off extra stain from the peel with distilled water
II Clean slide and cover slip before use
III Put only a drop of glycerin on the cover slip
IV Pull out a thin leaf peel V Use filter paper to remove extra stain from the peel
In the experiment to show that CO2 is given out during respiration, KOH is used to :
Which of the following precautions are to be taken to perform the experiment „To show that CO2 is given out during respiration.
(A) Conical flask should be air tight
(B) Seeds in the flask should be totally dry
(C) A small tube with freshly prepared KOH solution should be placed in the flask
(D) The end of the delivery tube should be above water level The correct answer is :
The chemical reaction between barium chloride solution and sodium sulphate solution is an example of :
A student determined the pH of the given four samples using pH paper.
Sodium bicarbonate Hydrochloric acid Lemon juice Distilled water
I II III IV
The pH paper will turn red/pink with the following :
A few drops of a liquid ‘A’ were added to distilled water. The water now turned pH paper blue. The liquid ‘A’ is –
A pinch of sodium carbonate was added to two test tubes – one containing dil HCl (A) and the other containing dilute NaOH (B). The correct observation was
Uma took an aluminium strip and dipped it in freshly prepared ferrous sulphate solution taken in a test tube. After some time, she observed some changes. One of her correct observations is :
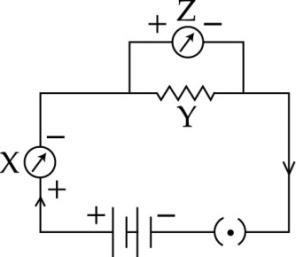
A student draws the following circuit diagram for the experiment ‘To study the dependence of V on current (I) across a resistor’. The parts labeled X, Y and Z in this diagram are respectively:
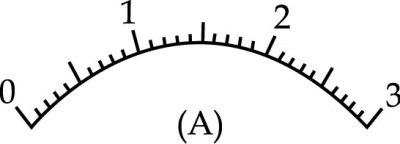
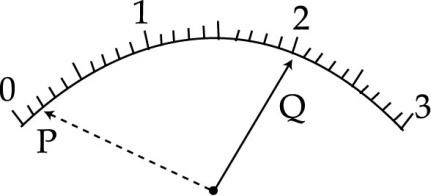
A student is performing the experiment to determine the equivalent resistance of two resistors when connected in parallel. She observes that the voltmeter pointer is at point ‘P’ when key is unplugged and the pointer is at Q when the key is plugged. The correct voltmeter reading is :
Choose the appropriate set of apparatus for performing the experiment for finding the equivalent resistance of two resistors when connected in series :
To make the leaves of a potted plant starch free, a student should –
A water bath must be used to boil leaf in ethanol because :