Sample AFMC Chemistry Mock Test - NEET MCQ
30 Questions MCQ Test AFMC Mock Test Series 2025 - Sample AFMC Chemistry Mock Test
Which of the following will not be soluble in sodium hydrogen carbonate ?
Reaction of tert-butyl bromide with sodium methoxide produces
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The segment of DNA which acts as the instrumental manual for the synthesis of protein is
A carboxylic acid is converted into its anhydride using
The geometric form of crystals is the result of orderly arrangement of
Just before attaining equilibrium by a reversible reaction, it is found that
For the reaction 2A + B → 3C + D. Which of the following does not express the reaction rate?
Which of the following favours the backward reaction in a chemical equilibrium?
Effect of temperature on rate of reaction given by
On Pauling scale which of the following does not have electronegativity ≥ 3.0
The heat of combustion of methane at 298K is expressed by CH4(g) + 2O2(g) → CO2(g) + 2H2O(l), ΔH = -890.2kJ. The magnitude of ΔE of the reaction at this temperature is
Which of the following electronic configuration corresponds to an inert gas?
A neutral fertilizer among the following compounds is
In hydrogen-oxygen fuel cell,combustion of hydrogen occurs to
At STP 1.12 litre of H2 is obtained on flowing a current for 965 seconds in a solution. The value of current is
Alkali metals impart colour to Bunsen flame due to
A hydrocarbon reacts with hypochlorous acid to give 2-chloroethanol. The hydrocarbon is
...... test is used for detecting unsaturation in hydrocarbons
A radioactive isotope decays at such a rate that after 96 minutes only 1/8 th of the original amount remains. The half-life of this nuclide in minutes is
|
1 docs|30 tests
|


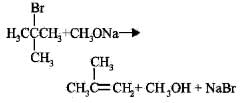 Thus, the reaction produces isobutylene.
Thus, the reaction produces isobutylene.














