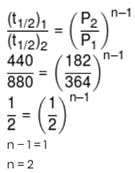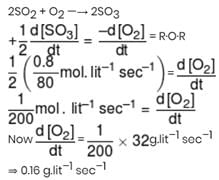Sample JEE Main Chemistry Mock Test - JEE MCQ
25 Questions MCQ Test - Sample JEE Main Chemistry Mock Test
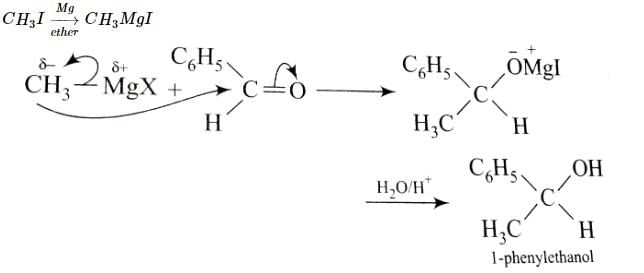
1-Phenylethanol can be prepared by the reaction of benzaldehyde with:
Phenols are more acidic than alcohols because ______.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If principal quantum no. of atom is 3, then its azimuthal quantum number is:
Electronic configuration of deuterium atom is:
The effect of atoms or groups loosing electrons towards a carbon atom is called:
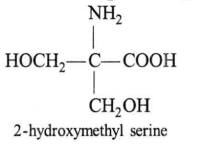
Among the following the achiral amino acid is:
Which of the following is paramagnetic species?
The IUPAC name for the complex
[Co(NO2) (NH3)5] Cl2 is :
How many EDTA (ethylenediaminetetraacetic acid) molecules are required to make an octahedral complex with a Ca2+ ion?
The correct IUPAC name of the structure given below is :
Low spin complexes are generally formed by the elements of:
When HCl gas is passed through a saturated solution of common salt, pure NaCl is precipitated because
Which of the following statement is correct with respect to property of elements with an increase in atomic no. in the carbon family?
A non-volatile electrolyte dissolved in an aqueous solution in same molal proportion as non-electrolyte produces ______.
Which of the following alkali metals has the highest tendency for the half cell reaction?
M(g) → M(g)+ + e-
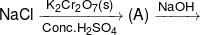
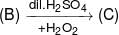
Determine total number of atoms in per unit formula of (A), (B) & (C).
4 mole of a mixture of Mohr's salt and Fe2(SO4)3 requires 500 mL of 1 M K2Cr2O7 for complete oxidation in acidic medium. The mole % of the Mohr's salt in the mixture is :-
The half-life of decomposition of gaseous CH3CHO at initial pressure of 364 mm and 182 mm of Hg were 440 sec and 880 sec respectively. The order of the reaction is :-
If rate of formation of SO3 is 0.8 g.lit–1.sec–1 then calculate the rate of disappearance of O2 in g.lit–1.sec–1 for the reaction 2SO2 + O2 → 2SO3
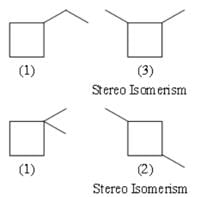
The total number of possible isomers with molecular formula C6H12 that contain a cyclobutane ring.


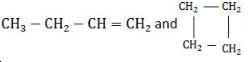
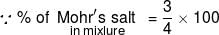 = 75
= 75