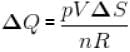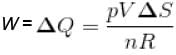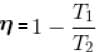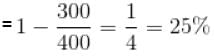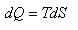Second Law Of Thermodynamics MCQ Level - 1 (Part - 1) - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - Second Law Of Thermodynamics MCQ Level - 1 (Part - 1)
A sample of an ideal gas with initial pressure p and volume V is taken through an isothermal expansion proceed during which the change in entropy is found to be ΔS. The universal gas constant is R. Then, the work done by the gas is given by :
A Carnot engine operating between 27°C and 127°C has efficiency equal to
Select one:
Select one:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
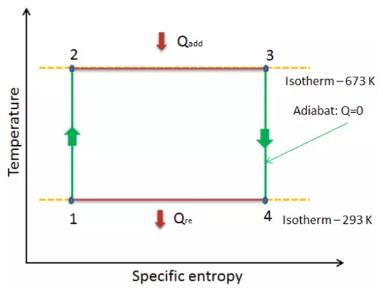
T-S diagram for a Carnot’s cycle is
Select one:
Select one:
The entropy of an isolated system
Select one:
A Carnot cycle operates on a working substance between two reservoirs at temperatures T1 and T2 , where, T1 > T2 . Amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements is incorrect?
Two ends of a rod are kept at 127°C and 227°C. When 2000 cal of heat flows in this rod, then the change in entropy is
Select one:
At 0 K, fluids are assumed to have
Select one:
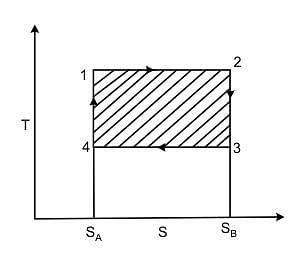
The area of the Carnot cycle on a T-S diagram represents
Select one:
A reversible heat engine can have 100% efficiency if the temperature of sink is
Select one:
Which of the following is a property of entropy?
Select one:


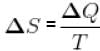 ...(ii)
...(ii)