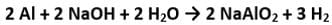States Of Matter : Gases & Liquids - Practice Test (1) - Class 9 MCQ
25 Questions MCQ Test - States Of Matter : Gases & Liquids - Practice Test (1)
The three important states of matter are
In the kinetic molecular theory attraction between molecules of the gas is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The drain cleaner, Drainex contains small bits of aluminum which react with caustic soda to produce dihydrogen. What volume of dihydrogen at 20 ∘C and one bar will be released when 0.15g of aluminum reacts?
A person living in Shimla observed that cooking food without using pressure cooker takes more time. The reason for this observation is that at high altitude:
Which of the following figures does not represent 1 mole of dioxygen gas at STP?
Physical behavior of the states differs greatly even though chemical behavior of the three states is identical because
Real gases show deviations from ideal gas law because
What will be the pressure exerted by a mixture of 3.2 g of methane and 4.4 g of carbon dioxide contained in a 9 dm3 flask at 27∘C ?
Which of the following property of water can be used to explain the spherical shape of rain droplets?
under which of the following two conditions, a gas deviates most from the ideal behavior?
dipole-dipole forces act between molecules that have
Which is the correct form of van der Waals equation?
What will be the pressure of the gaseous mixture when 0.5 L of H2 at 0.8 bar and 2.0 L of dioxygen at 0.7 bar are introduced in a 1L vessel at27∘C ?
A plot of volume (V ) versus temperature (T ) for a gas at constant pressure is a straight line passing through the origin. The plots at different values of pressure are shown in Figure. Which of the following order of pressure is correct for this gas?
Which of the following changes decrease the vapour pressure of water kept in a sealed vessel?
Which of the following statements about Hydrogen bond incorrect?
behaviour of the gas becomes more ideal when
Density of a gas is found to be 5.46 g/dm3g/dm3 at 27 ∘C∘C at 2 bar pressure. What will be its density at STP?
The interaction energy of London force is inversely proportional to sixth power of the distance between two interacting particles but their magnitude depends upon
Heat of vaporization is always larger than the Heat of fusion because
The intermolecular force primarily responsible for the condensed states of nonpolar substances is the
Critical temperature (TC)of carbon dioxide is the highest temperature at which liquid carbon dioxide is observed. Above this temperature
34.05 mL of phosphorus vapour weighs 0.0625 g at 546∘C and 0.1 bar pressure. What is the molar mass of phosphorus?
Dipole-dipole forces act between the molecules possessing permanent dipole. Ends of dipoles possess ‘partial charges’. The partial charge is
The fraction of molecules with enough energy to escape the liquid