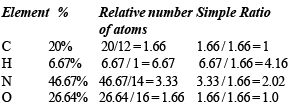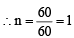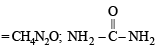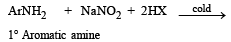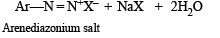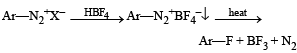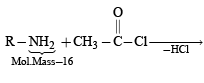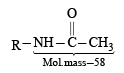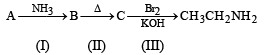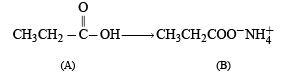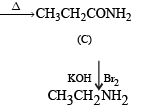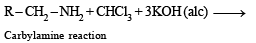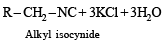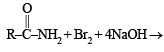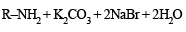Test: 35 Year JEE Previous Year Questions: Compounds Containing Nitrogen - JEE MCQ
26 Questions MCQ Test 35 Years Chapter wise Previous Year Solved Papers for JEE - Test: 35 Year JEE Previous Year Questions: Compounds Containing Nitrogen
PASSAGE - 1
The conversion of an amide to an amine with one carbon atom less by the action of alkaline hydrohalite is known as Hofmann bromamide degradation.
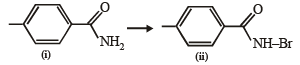
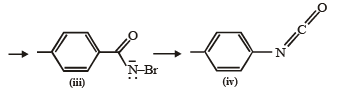
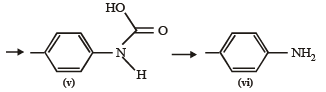
In this reaction, RCONHBr is formed from which the reaction has derived its name. Hofmann reaction is accelerated if the migrating group is more electron-releasing. Hofmann degradation reaction is an intramolecular reaction.
Q. How can the conversion of (i) to (ii) be brought about?
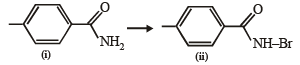
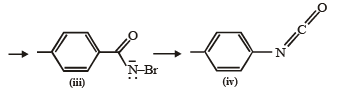

PASSAGE - 1
The conversion of an amide to an amine with one carbon atom less by the action of alkaline hydrohalite is known as Hofmann bromamide degradation.
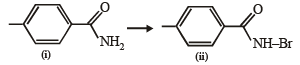
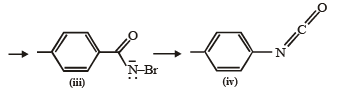

In this reaction, RCONHBr is formed from which the reaction has derived its name. Hofmann reaction is accelerated if the migrating group is more electron-releasing. Hofmann degradation reaction is an intramolecular reaction.
Q. Which is the rate determining step in Hofmann bromamide degradation?
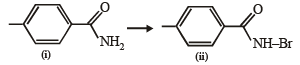
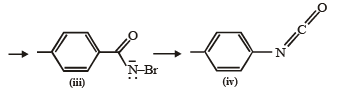

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
PASSAGE - 1
The conversion of an amide to an amine with one carbon atom less by the action of alkaline hydrohalite is known as Hofmann bromamide degradation.
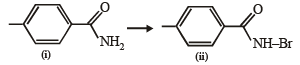
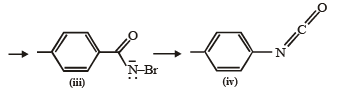

In this reaction, RCONHBr is formed from which the reaction has derived its name. Hofmann reaction is accelerated if the migrating group is more electron-releasing. Hofmann degradation reaction is an intramolecular reaction.
Q. What are the constituent amines formed when the mixture of (i) and (ii) undergoes Hofmann bromamide degradation?
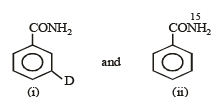
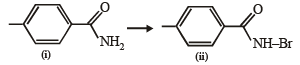
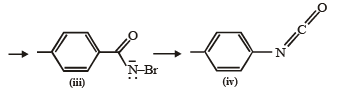

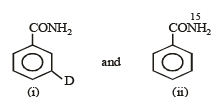
PASSAGE - 2
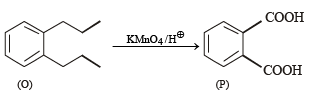
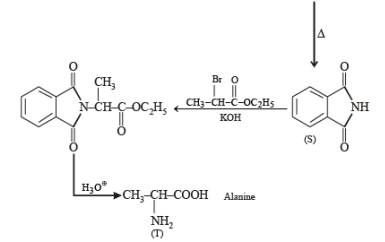
Treatment of compound O with KMnO4/H+ gave P, which on heating with ammonia gave Q. The compound Q on treatment with Br2/NaOH produced R. On strong heating, Q gave S, which on further treatment with ethyl 2-bromopropanoate in the presence of KOH followed by acidification, gave a compound T.
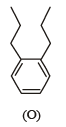
Q. The compound R is
PASSAGE - 2
Treatment of compound O with KMnO4/H+ gave P, which on heating with ammonia gave Q. The compound Q on treatment with Br2/NaOH produced R. On strong heating, Q gave S, which on further treatment with ethyl 2-bromopropanoate in the presence of KOH followed by acidification, gave a compound T.

Q. The compound T is
Read the following Statement-1(Asseration) and Statement -2 (Reason) and answer as per the options given below :
Q.
Statement - 1: p-Nitrophenol is a stronger acid than o-nitrophenol.
Statement - 2 : Intramolecular hydrogen bonding makes the o-isomer weaker than the p-isomer.
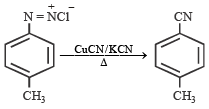
Statement - 1: Benzonitrile is prepared by the reaction of chlorobenzene with potassium cyanide.
Statement - 2 : Cyanide (CN–) is a strong nucleophile.
Statement - 1 : In strongly acidic solutions, aniline becomes more reactive towards electrophilic reagents.
Statement-2 : The amino group being completely protonated in strongly acidic solution, the lone pair of electrons on the nitrogen is no longer available for resonance.
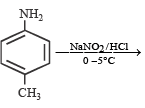
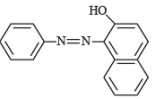
Statement - 1 : Aniline on reaction with NaNO2 / HCl at 0oC followed by coupling with β-naphthol gives a dark blue precipitate. and
Statement - 2 : The colour of the compound formed in the reaction of aniline with NaNO2/HCl at 0oC followed by coupling with β-naphthol is due to the extended conjugation.
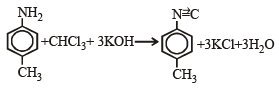
When primary amine reacts with chloroform in ethanolic KOH then the product is
The reaction of chloroform with alcoholic KOH and p-toluidine forms
The correct order of increasing basic nature for the bases NH3, CH3NH2 and (CH3)2 NH is
Ethyl isocyanide on hydrolysis in acidic medium generates
Which one of the following methods is neither meant for the synthesis nor for separation of amines?
Amongst the following the most basic compound is
An organic compound having molecular mass 60 is found to contain C = 20%, H = 6.67% and N = 46.67% while rest is oxygen. On heating it gives NH3 alongwith a solid residue.
The solid residue give violet colour with alkaline copper sulphate solution. The compound is
Which one of the following is the strongest base in aqueous solution ?
In the chemical reaction,
CH3CH2NH2 + CHCl3 + 3KOH → (A) + (B) + 3H2O, the compounds (A) and (B) are respectively
In the chemical reactions,
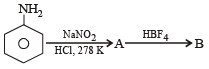
the compounds ‘A’ and ‘B’ respectively are
A compound with molecular mass 180 is acylated with CH3COCl to get a compound with molecular mass 390. The number of amino groups present per molecule of the former compound is :
An organic compound A upon reacting with NH3 gives B. On heating B gives C. C in presence of KOH reacts with Br2 to given CH3CH2NH2. A is :
The gas leaked from a storage tank of the Union Carbide plant in Bhopal gas tragedy was :
On heating an aliphatic primary amine with chloroform and ethanolic potassium hydroxide, the organic compound formed is:
Considering the basic strength of amines in aqueous solution, which one has the smallest pKb value?
In the reaction
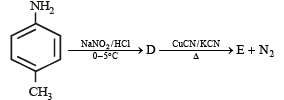
the product E is :
In the Hofmann bromamide degradation reaction, the number of moles of NaOH and Br2 used per mole of amine produced are :
|
347 docs|185 tests
|
|
347 docs|185 tests
|


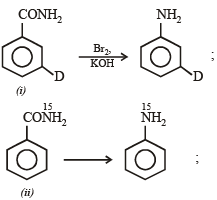
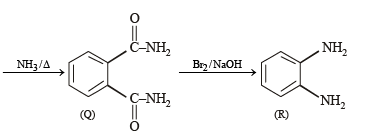
 group exerts strong –I effect causing deactivation of the
group exerts strong –I effect causing deactivation of the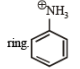
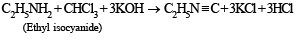

 is most basic. Inothers the basic character is suppressed due to Resonance (see applications of resonance).
is most basic. Inothers the basic character is suppressed due to Resonance (see applications of resonance).