Test: Aldehydes and Ketones - 2 - MCAT MCQ
10 Questions MCQ Test Organic Chemistry for MCAT - Test: Aldehydes and Ketones - 2
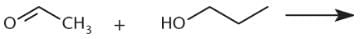
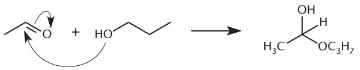
Which of the following reactions would produce the compound below?
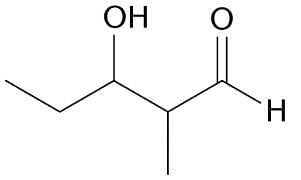

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
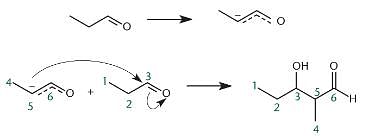
The aldol condensation is an example of which reaction type(s)?
I. Dehydration
II. Cleavage
III. Nucleophilic addition
I. Dehydration
II. Cleavage
III. Nucleophilic addition
When reacted with ammonia (NH3) at 200°C, which enolate of a carbonyl-containing compound would predominate?
α-hydrogens of a ketone are acidic due to:
I. resonance stabilization.
II. the electron-withdrawing properties of the alkyl groups.
III. the electronegative carbonyl oxygen.
When succinaldehyde is treated with lithium diisopropylamide (LDA), it:
I. becomes more nucleophilic.
II. becomes less nucleophilic.
III. generates a carbanion.
When benzaldehyde is reacted with acetone, which will act as the nucleophile?
The catalytic production of dihydroxyacetone and glyceraldehyde 3-phosphate (2-hydroxy-3-oxopropyl dihydrogen phosphate) from fructose-1,6-bisphosphate ({[(2S,3S,4S,5R)-3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]methoxy}phosphonic acid) is what type of reaction?
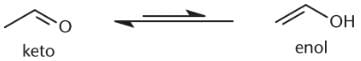
Why does the equilibrium between keto and enol tautomers lie far to the keto side?
I. The keto form is more thermodynamically stable.
II. The enol form is lower energy.
III. The enol form is more thermodynamically stable.
|
140 videos|5 docs|15 tests
|
|
140 videos|5 docs|15 tests
|