Test: Aromatic Electrophilic Substitution - JEE MCQ
20 Questions MCQ Test - Test: Aromatic Electrophilic Substitution
Direction (Q. Nos. 1 - 10) This is section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d) out of which ONLY ONE option is correct.
Q. The electrophile in sulphonation of benzene using fuming sulphuric acid is
In nitration of benzene using cone. H2SO4 and cone. HNO3, the role of nitric acid is
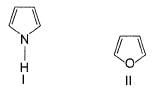
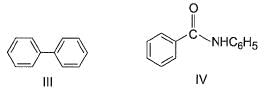
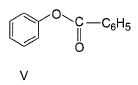
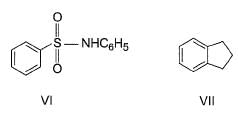
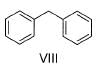
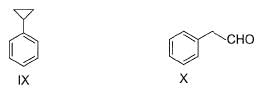
The increasing order of reactivity of the following compounds towards electrophilic aromatic substitution reaction is
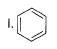
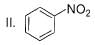
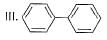
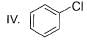




What is the correct increasing order of reactivity of the following compounds towards electrophilic aromatic substitution reaction?
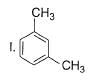
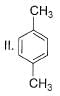
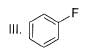
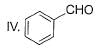
What is major product of the reaction below?
Predict major product of the following reaction.
Which of the following act as electrophile in halogenation?
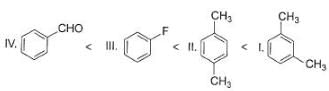
Identify the correct order of reactivity of following towards aromatic electrophilic substitution reaction.
I. Toluene
II. Benzene
III. Chlorobenzene
IV. Nitrobenzene
Predict the major product in the following reaction.
[IIT JEE 2001]
What is true about sulphonation of C6H6?
Direction (Q. Nos. 11 - 16) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage I
Consider the following road-map reaction,
Q. The most likely structure of A is
Passage I
Consider the following road-map reaction,
Q. What is correct about D?
Passage I
Consider the following road-map reaction,
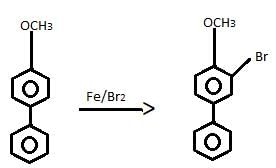
Q. If A is treated with cone. HNO3 and cone. H2SO4 mixture, mononitration at phenyl ring takes place. What is the structure of major nitration product?
Passage II
Consider the following road-map reaction,
Q. The most likely structure of A is
Passage II
Consider the following road-map reaction,
Q. What is true about C?
Passage II
Consider the following road-map reaction,
Q. What is the structure of E?
Direction (Q. No. 22 - 25) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q. C6H4Br2(P) is a dibromo benzene and it is the less polar isomer of its two polar isomers. If P is treated with conc. HNO3 and conc -H2SO4 mixture, in principle, how many mono nitration products are expected?
How many isomers of C7H8O exists that has a phenyl ring?
If the following compound is treated with Br2-Fe, how many mono bromination products are formed in principle?
How many of the following have an activated aromatic ring for electrophilic substitution reaction?