Test: Atomic Physics - 4 - JEE MCQ
17 Questions MCQ Test - Test: Atomic Physics - 4
The distance of the closest approach of an alpha particle fired at a nucleus with momentum 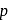 is
is 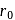 . The distance of the closest approach when the alpha particle is fired at the same nucleus with momentum
. The distance of the closest approach when the alpha particle is fired at the same nucleus with momentum 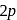 will be
will be
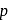 is
is 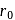 . The distance of the closest approach when the alpha particle is fired at the same nucleus with momentum
. The distance of the closest approach when the alpha particle is fired at the same nucleus with momentum 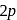 will be
will beThe ratio of the wavelengths of the longest wavelength lines in the Lyman and Balmer series of hydrogen spectrum is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which energy state of the triply ionized beryllium 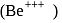 has the same electron orbital radius as that of the ground state of hydrogen? Given
has the same electron orbital radius as that of the ground state of hydrogen? Given 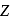 for beryllium
for beryllium 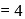
Which energy state of doubly ionized lithium (Li 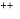 ) has the same energy as that of the ground state of hydrogen? Given
) has the same energy as that of the ground state of hydrogen? Given 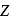 for lithium
for lithium 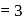 . What is the ratio of the electron orbital radius of
. What is the ratio of the electron orbital radius of 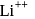 to that of hydrogen?
to that of hydrogen?
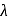 , the recoil speed of the atom of mass
, the recoil speed of the atom of mass 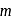 is given by
is given by
The energy required to excite a hydrogen atom from n = 1 to n = 2 energy state is 10.2eV. What is the wavelength of the radiation emitted by the atom when it goes back to its ground state?
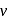 . What is the frequency of the corresponding line in the spectrum of doubly ionized Lithium?
. What is the frequency of the corresponding line in the spectrum of doubly ionized Lithium?
The ionization energy of hydrogen atom is 13.6eV. Hydrogen atoms in the ground state are excited by electromagnetic radiation of energy 12.1 eV. How many spectral lines will be emitted by the hydrogen atoms?
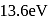 What is the ionization energy of helium atom?
What is the ionization energy of helium atom?
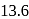 eV. Its energy in
eV. Its energy in 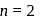 energy state is
energy state is
The distance of the closest approach of an alpha particle fired at a nucleus with kinetic energy 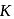 is
is 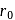 . The distance of the closest approach when the alpha particle is fired at the same nucleus with kinetic energy
. The distance of the closest approach when the alpha particle is fired at the same nucleus with kinetic energy 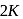 will be
will be
The total energy of the electron in the first excited state of hydrogen is 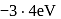 . What is the kinetic energy of the electron in this state?
. What is the kinetic energy of the electron in this state?
In the Bohr model of the hydrogen atom, the ratio of the kinetic energy to the total energy of the electron in a quantum state n is
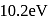 . What is the corresponding energy difference for a singly ionized helium atom?
. What is the corresponding energy difference for a singly ionized helium atom?
In Rutherford's alpha particle scattering experiment with thin gold foil, 8100 scintillations per minute are observed at an angle of 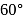 . The number of scintillations per minute at an angle of
. The number of scintillations per minute at an angle of 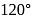 will be
will be
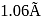 . What is the diameter of the tenth orbit?
. What is the diameter of the tenth orbit?
The total energy of the electron in the first excited state of hydrogen is 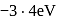 . The potential energy of the electron is
. The potential energy of the electron is


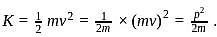 Therefore
Therefore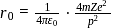
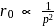 . When
. When 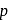 is doubled,
is doubled, 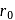 becomes onefourth. Hence the correct choice is (d).
becomes onefourth. Hence the correct choice is (d).
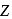 , the radius of the
, the radius of the 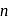 th orbit is given by [see Eq.]
th orbit is given by [see Eq.]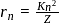 ...(i)
...(i)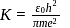 is a constant. For the ground state of hydrogen
is a constant. For the ground state of hydrogen 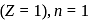 so that
so that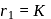
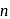 be the energy state of
be the energy state of 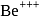 for which the orbital radius is
for which the orbital radius is 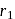 . Putting
. Putting 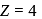 and
and 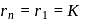 is Eq. (i) we get
is Eq. (i) we get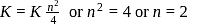
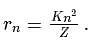 Therefore, for hydrogen (n = 1 state), we have
Therefore, for hydrogen (n = 1 state), we have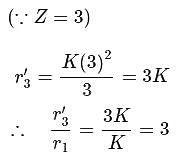
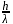 , from the law of conservation of momentum, the recoil speed
, from the law of conservation of momentum, the recoil speed 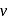 of an atom of mass
of an atom of mass 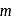 is given by
is given by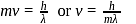
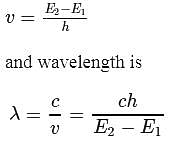
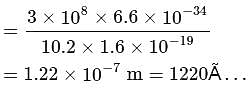
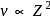 . For doubly ionized lithium
. For doubly ionized lithium 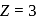 . Hence the correct choice is (c).
. Hence the correct choice is (c).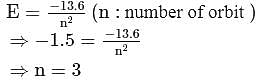
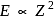 . For helium
. For helium 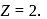 Hence
Hence 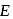 for helium
for helium 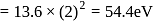 , which is choice (c).
, which is choice (c).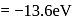 . Its value in the second orbit is
. Its value in the second orbit is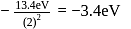
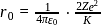
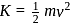 . Thus
. Thus 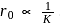 When
When 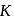 is doubled,
is doubled, 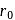 becomes half. Hence the correct choice is (c).
becomes half. Hence the correct choice is (c).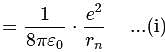
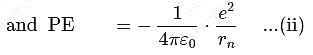
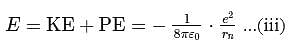
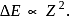 For a singly ionized helium atom
For a singly ionized helium atom 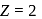 .
. 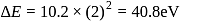
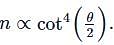 Hence
Hence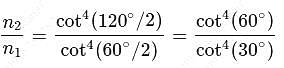
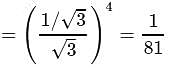
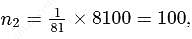 which is choice (a).
which is choice (a). th orbit is proportional to
th orbit is proportional to  , i.e.
, i.e. 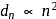 . Given
. Given 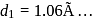 . Therefore
. Therefore 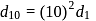
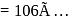 . Hence the correct choice is (d).
. Hence the correct choice is (d).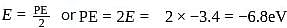 .
.














