Test: Atoms - NEET MCQ
30 Questions MCQ Test Physics Class 12 - Test: Atoms
According to ‘plum pudding model’ atoms on the whole are electrically neutral because
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What is the shortest wavelength present in the Paschen series of spectral lines?
Average angle of deflection of α-particles by a thin gold foil predicted by Thomson’s model is
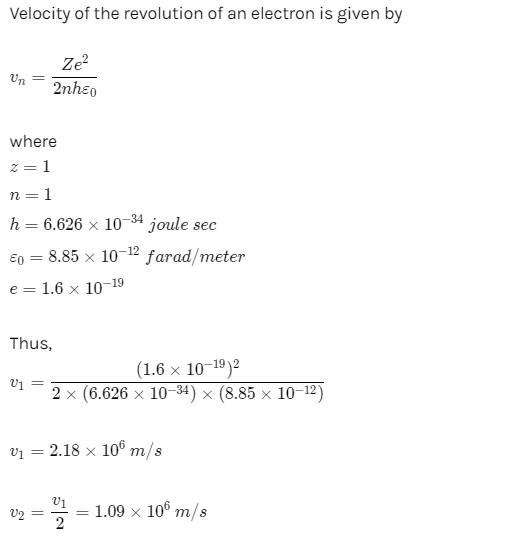
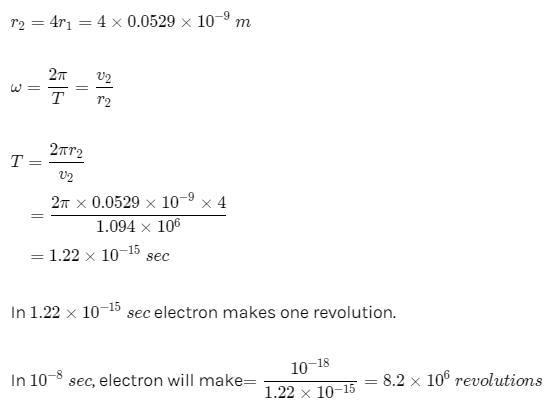
The average lifetime of the first excited level of a hydrogen atom is 1.0 ×10−8 s. In the Bohr model, how many orbits does an electron in the n = 2 level complete before returning to the ground level?
Which of these statements about Bohr model applied to hydrogen atom correct?
Probability of backward scattering (i.e., scattering of α -particles at angles greater than 90∘) predicted by Thomson’s model is
In Geiger-Marsden experiment very small deflection of the beam was expected because
Suppose you are given a chance to repeat the alpha-particle scattering experiment using a thin sheet of solid hydrogen in place of the gold foil. (Hydrogen is a solid at temperatures below 14 K.) What results do you expect?
It is found experimentally that for small thickness t, the number of α-particles scattered at moderate angles is proportional to t. What clue does this linear dependence on t provide?
An electron collides with a hydrogen atom in its ground state and excites it to a state of n = 3. How much energy was given to the hydrogen atom in this inelastic collision?
Which of these statements correctly describe the atomic model according to classical electromagnetic theory ?
In the ground state of which model electrons are in stable equilibrium with zero net force?
A triply ionized beryllium ion Be3+, (a beryllium atom with three electrons removed), behaves very much like a hydrogen atom except that the nuclear charge is four times as great. For the hydrogen atom, the wavelength of the photon emitted in the n =2 to n=1 to transition is 122 nm. What is the wavelength of the photon emitted when a Be3+ ion undergoes this transition?
Fluorescent lamps are more efficient than incandescent lamps in converting electrical energy to visible light because
In which of the models An atom has a nearly continuous mass distribution?
Find the longest wavelength present in the Balmer series of hydrogen, corresponding to the H- line.
The model that best explains the results of Geiger-Marsden experiment is
In a Geiger -Marsden experiment, what is the distance of closest approach d to the nucleus of a 7.7 MeV α−particle before it comes momentarily to rest and reverses its direction?
|
97 videos|336 docs|104 tests
|