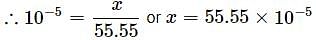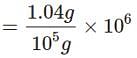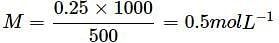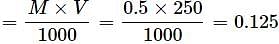Test: Basic Concepts of Solutions(17 Sep) - JEE MCQ
10 Questions MCQ Test Daily Test for JEE Preparation - Test: Basic Concepts of Solutions(17 Sep)
During dissolution when solute is added to the solvent, some solute particles separate out from the solution as a result of crystallisation. At the stage of equilibrium, the concentration of solute in the solution at given temperature and pressure
According to Henry's law the partial pressure of the gas in vapour phase (p) is proportional to the mole fraction of the gas (x) in the solution. For different gases the correct statement about Henry's constant is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
H2S is a toxic gas used in qualitative analysis. If solubility of H2S in water at STPSTP is 0.195m, what is the value of KH?
When a gas is bubbled through water at 298K, a very dilute solution of gas is obtained. Henry’s law constant for the gas is 100kbar. If gas exerts a pressure of 1bar1bar, the number of moles of gas dissolved in 11 litre of water is
What is the mole fraction of glucose in 10% w/W glucose solution?
When 1.04g of BaCl2 is present in 105g of solution, the concentration of solution is:
What is the molarity of a solution containing 10 g of NaOH in 500 mL of solution?
How many grams of NaOH are present in 250 mL of 0.5 M NaOH solution?
The density of a solution prepared by dissolving 120g of urea (mol. mass = 60u) in 1000g of water is 1.15g/mL. The molarity of this solution is
|
360 tests
|


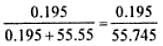
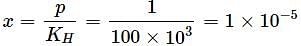
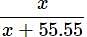 (55.55 >>> x)
(55.55 >>> x)