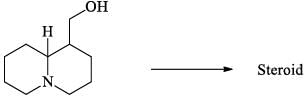Test: Biomolecules Level - 4 - Chemistry MCQ
30 Questions MCQ Test Organic Chemistry - Test: Biomolecules Level - 4
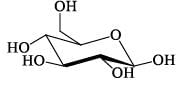
Which of the following compounds will form an osazone derivative?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
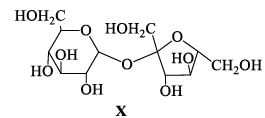
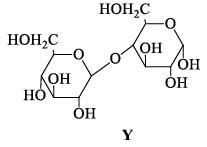
The correct statement(s) about the following sugars X and Y is/are:
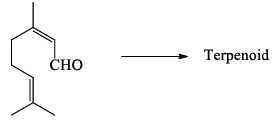
Consider the following reaction. 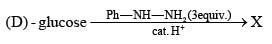
Among the following, the compound(s) whose osazone derivative(s) will have the same melting point as that of X is/are:
Which of the following are non-covalent interactions important is maintaining the secondary, tertiary and quaternary aspects of amino acids:
Which pair(s) of amino acids cannot have hydrophobic interactions:
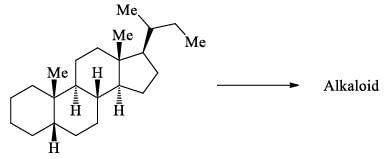
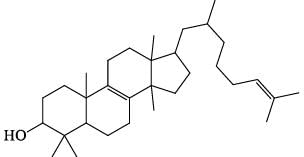
Which of the following regarding steroids is/are correct:
Which of the following statement regarding to properties of starch is/are correct:
Which of the following pair regarding to biological importance of carbohydrates is/are correctly matched:
Which of the following regarding to the examples of Keto sugar is/are correct:
Which of the following pairs is/are correctly matched:
D-Glucose exist in x different forms. The value of x(stereoisomer) is:
How many of the following carbohydrate would not undergo mutarotaion in aqueous solutions:
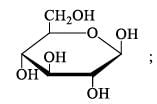
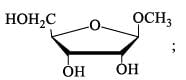
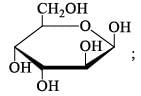
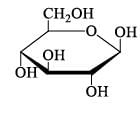
The number of reducing sugars among the following is…………….. [IIT JAM 2017]
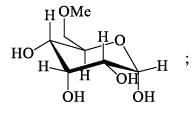
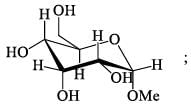
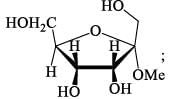
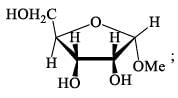
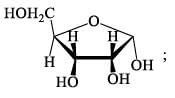
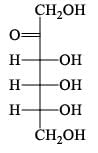
How many isophene unit are present in the following compound:
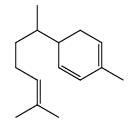
How many of the following will give same product on reaction with PhNHNH2 (excess):
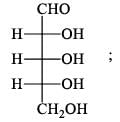
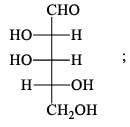
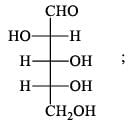
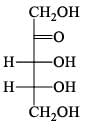
For 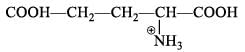 Glutamic acid value of pKa, pKa2, pKa3 are 2.00, 4.65 and 9.98 respectively. At which pH Glutomic acid will not be obtained during electroproresis at any one of the electrodes.
Glutamic acid value of pKa, pKa2, pKa3 are 2.00, 4.65 and 9.98 respectively. At which pH Glutomic acid will not be obtained during electroproresis at any one of the electrodes.
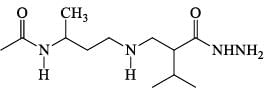
How many statements about peptides are correct.
(a) A dipeptide has two peptide linkage between amino acids.
(b) If only one amino group and one carboxylic acid group are available for the reaction, then only one dipeptide can form.
(c) By convention N-terminus is kept left and (–terminus) at siglate in the structure of a pepetide.
(d) A polypeptide with more than hundred amino acid residures (mal . mass > 10,000) is called a protein.
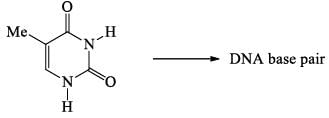
The number of hydrogen bond present in Guanine-Cytosine base pair is:
How many of the following are reducing sugar:
Sucrose, Lactose, Maltose, Glucose, Galactose, Fructose, Glycogen, Ribose.
How many different tripeptides can be obtained from alanine, glycine and phenylalanine, each tripeptide containing all the three amino acids?
Find the value of x + y for
Tyr-Ile-Gln-Arg-Leu-Gly-Phe-Lys-Asn-Trp-Pne-Gly-Ala-Lys-Gln-Gln-NH2
Where
X = Number of fragments obtained by the cleavage of Trypsin.
Y = Number of fragments obtained by the cleavage of chemotrpsin.
|
35 videos|92 docs|46 tests
|


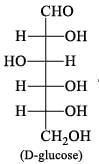 on epimerization with base gives
on epimerization with base gives