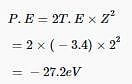Test: Bohrs Model of Atom (April 28) - NEET MCQ
10 Questions MCQ Test Daily Test for NEET Preparation - Test: Bohrs Model of Atom (April 28)
‘Hartree’ is the atomic unit of energy and is equal to
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Wave number of a spectral line for a given transition is x cm-1 for He+, then its value for Be3+ (isoelectronic of He+)for the same transition is
Which of the following electronic transitions requires that the greatest quantity of energy be absorbed by a hydrogen atom ?
An electron in H-atom in its ground state absorbs 1.5 times as much as energy as the minimum required for its escape from the atom
Q.
Thus, kinetic energy given to the emitted electron is
Ionisation energy of He+ is 19.6x10-18 J atom -1. The energy of the first stationary state (n = 1)of Li2+ is
The kinetic energy of an electron in the second Bohr orbit of a hydrogen atom is (a0 is Bohr radius)
[AIEEE 2012]
Energy of the electron in nth orbit is given by E Wavelength of light required to excite an electron in an H-atom from level n = 1 to n = 2 will be (h = 6.62 x 10-34 J s ; c = 3.0 x 108ms -1)
[AIEEE 2012]
The potential energy of an electron in the second Bohr's orbit of the he±
In Lyman series, shortest wavelength of H-atom appears at x m, then longest wavelength in Balmer series of He+ appear at
|
12 docs|366 tests
|