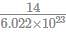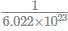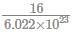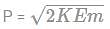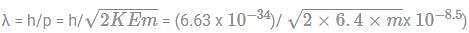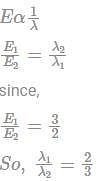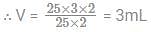Test: CSIR-NET Chemical Sciences Mock Test - 1 - UGC NET MCQ
30 Questions MCQ Test CSIR NET Exam Mock Test Series 2024 - Test: CSIR-NET Chemical Sciences Mock Test - 1
Gaurav spends 30% of his monthly income on food articles, 40% of the remaining on conveyance and clothes and saves 50% of the remaining. If his monthly salary is Rs. 18,400, how much money does he save every month ?
A shopkeeper cheats to the extent of 10% while buying and selling, by using false weights. His total gain is.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The greatest number which on dividing 1657 and 2037 leaves remainders 6 and 5 respectively, is:
If log 2 = 0.3010 and log 3 = 0.4771, the values of log5 512 is
A, B and C can do a piece of work in 24 days, 30 days and 40 days respectively. They began the work together but C left 4 days before the completion of the work. In how many days was the work completed?
The average weight of 8 persons increases by 2.5 kg when a new person comes in place of one of them weighing 65 kg. What might be the weight of the new person ?
A bag contains 4 white, 5 red and 6 blue balls. Three balls are drawn at random from the bag. The probability that all of them are red, is:
If two dice are thrown, then the probability that at least one of the dice shows a number less than 5 is:
In an exam, there were 5 questions. 10% of students solved all questions, 10% did not solve any question and 15% of the remaining students solved 1 question and 16% of total students solved 4 questions. If 24% of total students solved 2 questions and 140 students solved 3 questions, find the total number of students.
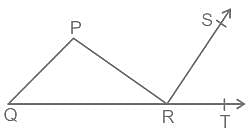
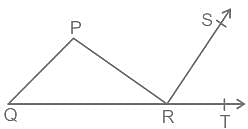
In the given figure, PQR is a triangle in which angle P : angle Q : angle R = 3 : 2 : 1, and PR is perpendicular to RS. What will be the measure of angle TRS?
If
- m + n + mn = 3
- n + p + np = 8
- m + p + pm = 15
then find the value of 12mnp
Pipe A can fill the tank in 6 hours and with help of B both can fill the tank in 12 hours. If both pipes opened together after how many hours should pipe B be closed so that the tank is filled in 8 hours?
A person divided an amount of Rs.100,000 into two parts and invested in two different schemes. In one he got 10% profit and in the other he got 12%. If the profit percentages are interchanged with these investments he would have got Rs.120 less. Find the ratio between his investments in the two schemes.
In a certain time a boat can cover 168 km downstream and in the same time it can cover 96 km upstream. If the speed of boat in still water is 11 km/h, then find the speed of current.
X and Y are two distinct digits, if the sum of the two-digit numbers formed by using both the digits is a perfect square, what could be the value of (X + Y)?
Give the correct order of initials T (true) or F (false) for following statements.
(i) If an ion has 2 electrons in K shell, 8 electrons in L shell and 6 electrons in M shell, then number of S electrons present in that element is 6.
(ii) The maximum number of electrons in a subshell is given by 2n2.
(iii) If electron has magnetic number –1, then it cannot be present in s-orbital.
(iv) Only one radial node is present in 3p orbital.
If 3.01× 1020 molecules are removed from 98 mg of H2SO4 then number of moles of H2SO4 left are
The number of atoms in 29gm of butane is :
Which of the following is the correct statement regarding the nitrogen in pyridine?
Glycerol on treatment with oxalic acid at 110° forms
Arrange the following in order of decreasing mass
i. 1F atom
ii. 1 N atom
iii. 1 O atom
iv. 1 H atom
If 40 ml of 0.2 M KOH is added to 160 ml of 0.1 M HCOOH [Ka = 2×10-4], the pOH of the resulting solution is
The crystal field splitting energy for tetrahederal (Δt) and octahederal (Δo) complexes are related as:
What is the value of de-broglie wavelength of electron accelerated at 400V?
If the energies of the two photons are in the ratio of 3 : 2, their wavelength will be the ratio of
The process of separating a crystalloid from a colloid by filtration is called-
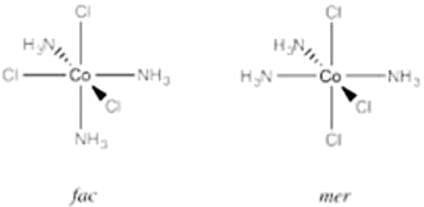
Which of the following compounds can exhibit fac - mer isomerism?
The volume of 0.25 M H3PO3 required to neutralise 25 ml of 0.03 M Ca(OH)2 is


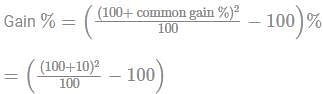
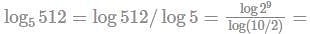

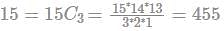
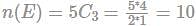
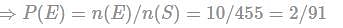
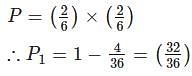
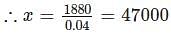
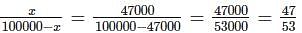
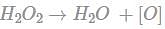
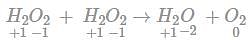
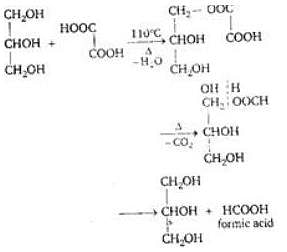
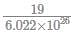 (since At.wt. of F = 19)
(since At.wt. of F = 19)