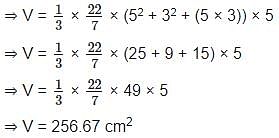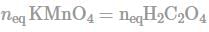Test: CSIR-NET Chemical Sciences Mock Test - 2 - UGC NET MCQ
30 Questions MCQ Test CSIR NET Exam Mock Test Series 2024 - Test: CSIR-NET Chemical Sciences Mock Test - 2
How many similiar words can be made by the words 'AERT' while every word should be used only one time.
How many letters are there in english alphabet in between 8 letter from left and 7 letters from right ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In a certain code language NOIDA is written as STNIF. How will MEERUT will be written in that code language ?
Find out the word from the following which can be formed by using the following letters.
VENTURESOME
Which substances turn red litmus paper into blue?
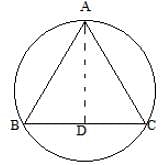
In the given figure AB =9m, AC = 11cm and the perpendicular AD = 4 cm. Find out the radius of the circumcircle.
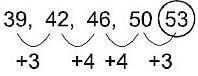
What is the next number in the following sequence?
39, 42, 46, 50, ...
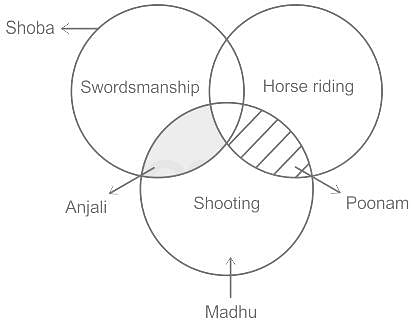
Shoba is good at swordsmanship. Poonam is good at horse riding and shooting but not good at swordsmanship. Madhu is good at shooting. Anjali is good at shooting and swordsmanship. Who is good at horse riding and shooting not swordsmanship?
The difference (in Rs.) between a discount of 35% on Rs. 3,600 and two successive discounts of 30% and 5% on the same amount is:
Find the value of ∠P + ∠Q + ∠R + ∠S + ∠T + ∠U in the figure shown below:

A thief steals a scooter at 11.00 AM, and drives it at a speed of 50 km/h. At 2 : 30 PM, the theft is detected and the owner of the scooter goes in a car traveling at 80 km/h in the same direction to catch the thief. At what time will the owner overtake (catch) the thief?
The current ages of Sudhir and Ashish are in the ratio 5 ∶ 7. Twelve years ago, the ratio of their ages was 1 ∶ 2. What will be the age of Sudhir after five years from now?
A dishonest shopkeeper promise to sell his goods at its C.P but he uses 20% less weight. Find profit %?
The frustum of a right circular cone has base diameter 10 cm, and on the top the diameter is 6 cm, and its height is 5 cm. Calculate its volume. (Correct to two decimal place) (usе π = 22/7)
A student is asked to multiply a number K by 5. But he multiplies K by 1/5 and gets x% error. Next time he multiplies K by 1/25 and gets y% error. Then the percentage increase of y over x is
Which of the region of IR spectra appears between (1400-600) cm-1 ?
Which of the following statement is incorrect about the SBR?
How many number of moles of KMnO4 will react with 180 gm H2C2O4 according to given reaction?
KMnO4 + H2C2O4 → 2CO2 + Mn2+
A cylinder contains 8 gms of He, 40 gms of Ne and 80 gms of Ar. (Molecular weights of the components are 4, 20 and 40 respectively)
How many moles of Ne are there in the cylinder?
The B.E.T. theory was proposed in the year
According to Faraday’s first law of electrolysis, the amount of any substance deposited at the electrode is directly proportional to the quantity of
Select the incorrect statement from the following option.
Which of the following act as a catalyst in the preparation of Buna-S?
Select the incorrect statement regarding analytical balance
Reactions which are bimolecular yet following ___________ order kinetics are called pseudo unimolecular reaction.
The unit of extent of a reaction is
The main cause of molecular aggregation is
Excess of KI reacts with CuSO4 solution and then Na2S2O3 solution is added to it. Which of the statement is incorrect for this reaction?
Chromatography involves two mutually