Test: Carbon and its compounds (Hard) - Class 10 MCQ
20 Questions MCQ Test Extra Documents, Videos & Tests for Class 10 - Test: Carbon and its compounds (Hard)
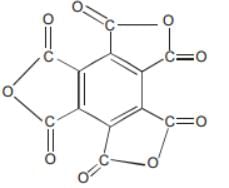
The functional group present in a molecule having the formula C12O9 is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
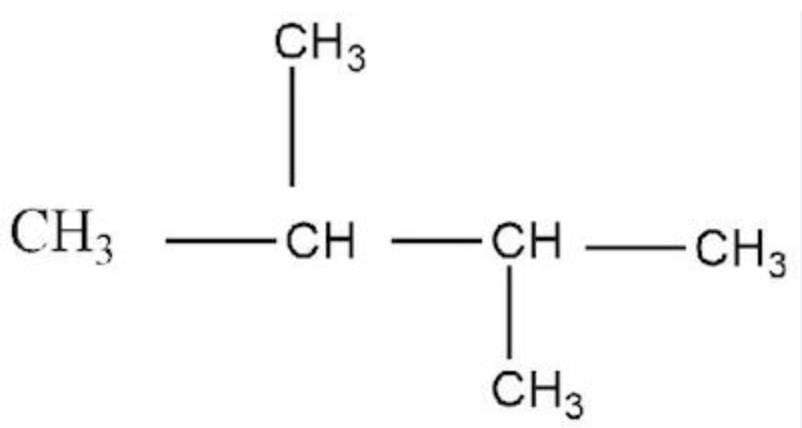
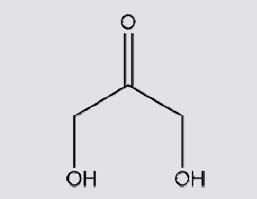
Select correct IUPAC naming of given compound
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that:
Which of the following compounds of carbon does not consist of ions?
The property of self-linkage among identical atoms to form long-chain compounds is known as:
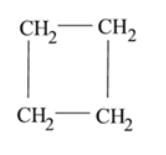
Which of the following is the molecular formula of cyclobutane?
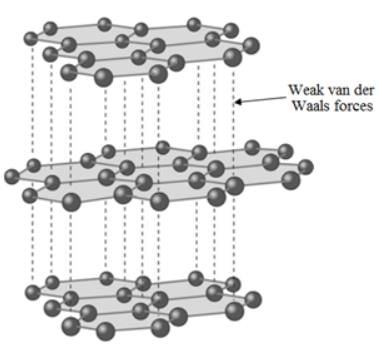
Which of the following statements about graphite and diamond is true?
How many number of carbon atoms are joined in a spherical molecule of buckminsterfullerene?
Which of the following is the major constituent of the liquefied petroleum gas?
The organic compounds having functional group are known as:
From which of the following substance pencil lead is formed?
Which of the following substance produces brisk effervescence with baking soda solution?
Which Of The Following Represents Saponification Reaction?
Which Of The Following Does Not Belong To The Same Homologous Series?
In The Last Year Board Examination, Rahul Were Asked A Question Where He Had To Choose The Statement Which Was/Were Incorrect? Will You Be Able To Answer This Question?
|
5 videos|292 docs|59 tests
|
|
5 videos|292 docs|59 tests
|