Test: Conformational Analysis - 2 - JEE MCQ
14 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Conformational Analysis - 2
Direction (Q. Nos. 1- 8) This section contains 8 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q.
Among the butane conformers, when viewed about C2-C3 bond, which occur at energy minima on a graph of potential energy versus dihedral angle?
choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
The correct statement regarding cyclopropane is/are
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Select the correct statement(s) concerning conformers of ethane and hexachloroethane?
The correct statement concerning various conformers of 2-fluoroethanol is/are
Consider the various conformers possible for meso form and enantiomeric 1,2-dibromo-1,2-dichloroethane and select the correct statement(s).
Select the correct statement(s) regarding conformers of 1,2-dimethyl cyclohexane.
The correct statement regarding conformation in butane is/are
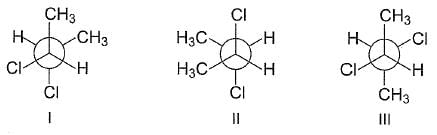
the correct statement(s) concerning the following Newmann's projection is/are
Direction (Q. Nos. 9-14) This section contains 2 paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)
Passage I
The two major contributors of conformers of 1,2-dichloroethane are anti and gauche. At 32°C in gas phase, the measured dipole moment of 1,2-dichloroethane is 1.12 D. The dipole moment of a mixture of X and Y is given by the relationship
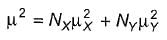
Here, N = mole fraction of each kind of molecule. From bond moment measurement, it has been estimated that gauche conformer of 1,2-dichloroethane should have a dipole moment of about 3.2 D.
Q.
What per cent of conformers of 1,2-dichloroethane is anti, at 32°C?
Passage I
The two major contributors of conformers of 1,2-dichloroethane are anti and gauche. At 32°C in gas phase, the measured dipole moment of 1,2-dichloroethane is 1.12 D. The dipole moment of a mixture of X and Y is given by the relationship
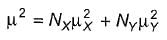
Here, N = mole fraction of each kind of molecule. From bond moment measurement, it has been estimated that gauche conformer of 1,2-dichloroethane should have a dipole moment of about 3.2 D.
Q.
What is true about percentage of gauche conformer?
Passage I
The two major contributors of conformers of 1,2-dichloroethane are anti and gauche. At 32°C in gas phase, the measured dipole moment of 1,2-dichloroethane is 1.12 D. The dipole moment of a mixture of X and Y is given by the relationship
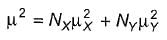
Here, N = mole fraction of each kind of molecule. From bond moment measurement, it has been estimated that gauche conformer of 1,2-dichloroethane should have a dipole moment of about 3.2 D.
Q.
What happens if temperature if increased to 52°C?
Passage II
Among conformers, the barrier to rotation originate mainly from torsional strain when atoms or groups eclipse one another. Followings are the costs of energy required to cross the rotational barrier for the indicated pair of groups eclipsing
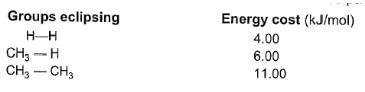
Q.
What is the energy barrier to rotation in neopentane ?
Passage II
Among conformers, the barrier to rotation originate mainly from torsional strain when atoms or groups eclipse one another. Followings are the costs of energy required to cross the rotational barrier for the indicated pair of groups eclipsing
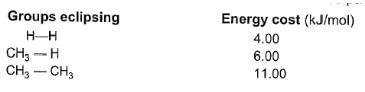
Q.
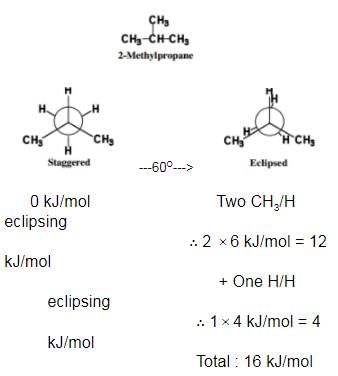
What is the energy barrier to rotation in 2-methyl propane ?
Passage II
Among conformers, the barrier to rotation originate mainly from torsional strain when atoms or groups eclipse one another. Followings are the costs of energy required to cross the rotational barrier for the indicated pair of groups eclipsing

Q.
What is the energy difference between two eclipsed conformers of butane when viewed between C2-C3 bond ?
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|