Test: Crystal Field Theory & Coloured Complexes (January 2) - NEET MCQ
10 Questions MCQ Test Daily Test for NEET Preparation - Test: Crystal Field Theory & Coloured Complexes (January 2)
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Among the following complexes the one which shows zero crystal field stabilisation energy is
The crystal field splitting energy (Δo) of
I. [CoBr6]3- II. [CoF6]3-
III. [Co(NCS)6]3- IV. [Co(CN)6]3- is in the order of
III. [Co(NCS)6]3- IV. [Co(CN)6]3- is in the order of
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The increasing order of wavelength of absorption for the complex ions
I. [Cr(NH3)6]3+
II. [CrCI6]3-
III. [Cr(H2O)6]3+
IV. [Cr(CN)6]3-
is :
IV. [Cr(CN)6]3-
CFSE is zero for the complexes with
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have.
Passage
In octahedral complexes due to repulsion between the ligands and d-orbitals, there is splitting of d-orbitals into two sets, i.e. two orbitals of higher energy called eg and three orbitals of lower energy called t2g. The difference of energy between the two sets of d-orbitals is called crystal field stabilisation energy denoted by Δo. For any given metal cation, the magnitude of Δo depends on the nature of ligands.
Q.
The CFSE for d7 configuration for strong ligand field is
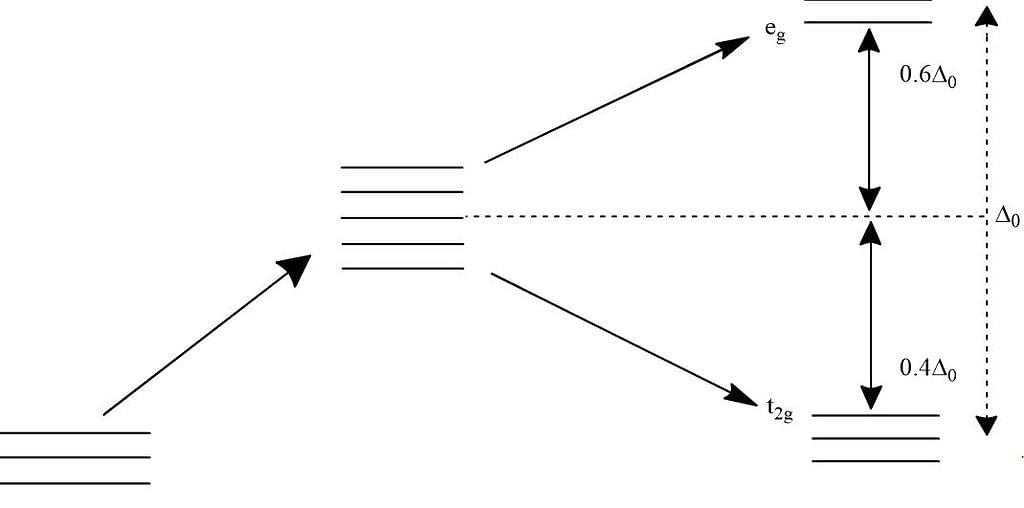
In octahedral complexes due to repulsion between the ligands and d-orbitals, there is splitting of d-orbitals into two sets, i.e. two orbitals of higher energy called eg and three orbitals of lower energy called t2g. The difference of energy between the two sets of d-orbitals is called crystal field stabilisation energy denoted by Δo. For any given metal cation, the magnitude of Δo depends on the nature of ligands.
Q.
In the following complexes of manganese, the distribution of electrons in d-orbitals of manganese
i. [Mn(H2O)6]2+
ii. [Mn(CN)6]4-
The number of ligands which have strong crystal field splitting than
H2O among SCN-, NCS-, EDTA4- , ,
, Br-, PPh3, F-
The total number of unpaired electrons in the two complexes [Cr(H2O)6]2+ and [Cr(CN)6]4- having octahedral geometry are
Number of unpaired electrons in t2g and eg orbitals in weak octahedral ligand fields with d7 configuration.
The crystal field splitting energies (CFSE) of high spin and low spin d6 metal complexes in octahedral complex in terms of Δo respectively are
|
12 docs|366 tests
|


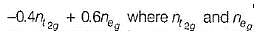 are the number of electron occupying the t2g and eg orbitatls respectively.
are the number of electron occupying the t2g and eg orbitatls respectively.














