JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Test: Cubic system - JEE MCQ
Test: Cubic system - JEE MCQ
Test Description
10 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Cubic system
Test: Cubic system for JEE 2024 is part of Chemistry for JEE Main & Advanced preparation. The Test: Cubic system questions and answers have been
prepared according to the JEE exam syllabus.The Test: Cubic system MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Cubic system below.
Solutions of Test: Cubic system questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Test: Cubic system solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Cubic system | 10 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Test: Cubic system - Question 1
The number of atoms in 100 g of an fcc crystal with density, d = 10 g/cm3 and cell edge equal to 100pm, is equal to
Detailed Solution for Test: Cubic system - Question 1
Test: Cubic system - Question 2
In the solid state,  has the same structure as that of sodium chloride. The number of oxygens surrounding each magnesium in
has the same structure as that of sodium chloride. The number of oxygens surrounding each magnesium in  is
is
Detailed Solution for Test: Cubic system - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Cubic system - Question 3
A mineral having the formula 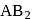 crystallizes in ccp lattice with
crystallizes in ccp lattice with  atoms occupying the lattice points. Pick out the correct statement of the following
atoms occupying the lattice points. Pick out the correct statement of the following
Detailed Solution for Test: Cubic system - Question 3
Test: Cubic system - Question 4
A metal has bcc structure and the edge length of its unit cell is 3.04A. The volume of the unit cell in cm3 will be
Detailed Solution for Test: Cubic system - Question 4
Detailed Solution for Test: Cubic system - Question 5
Test: Cubic system - Question 6
In a face centered cubic lattice atoms A are at the corner points and atoms B at the face centered points. If atom B is missing from one of the face centered points, the formula of the ionic compound is:
Detailed Solution for Test: Cubic system - Question 6
Test: Cubic system - Question 7
When molten zinc is converted into solid state, it acquires hcp structure. The number of nearest neighbours of 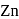 will be
will be
Detailed Solution for Test: Cubic system - Question 7
Test: Cubic system - Question 8
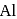 (at.
(at.  ) crystallizes in the cubic system with a cell edge of
) crystallizes in the cubic system with a cell edge of  . Its density is
. Its density is  per
per  Calculate the radius in
Calculate the radius in 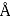 of the
of the  atom.
atom.
Detailed Solution for Test: Cubic system - Question 8
Detailed Solution for Test: Cubic system - Question 9
Test: Cubic system - Question 10
The intermetallic compound LiAg crystallizes in a cubic lattice in which both lithium and silver atoms have coordination number of eight. To what crystal class does the unit cell belong?
Detailed Solution for Test: Cubic system - Question 10
|
352 videos|596 docs|309 tests
|
Information about Test: Cubic system Page
In this test you can find the Exam questions for Test: Cubic system solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Cubic system, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF



 has a rock salt structure. In this structure each cation is surrounded by six anions and vice versa
has a rock salt structure. In this structure each cation is surrounded by six anions and vice versa

 is correct.
is correct. and that of fcc is also
and that of fcc is also  . It is higher in comparison to packing efficiency of simple cube and bcc which have values 52.4 and
. It is higher in comparison to packing efficiency of simple cube and bcc which have values 52.4 and  respectively.
respectively.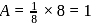 Number of atoms
Number of atoms 
 ,
,


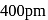 . Its body diagonal would be
. Its body diagonal would be is given by,
is given by, 
 , we get
, we get 















