Test: Dual Nature of Matter (April 23) - JEE MCQ
10 Questions MCQ Test Daily Test for JEE Preparation - Test: Dual Nature of Matter (April 23)
The threshold frequency v0 for a metal is 7.0 x 1014 s-1. Radiation of frequency v = 1.0 x 1015 s-1 hits the metal. Kinetic energy of the emitted electron is
Which of the following rules/principles is responsible to rule out the existence of definite paths or trajectories of electrons?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In an atom, an electron is moving with a speed of 600 ms-1 with an accuracy of 0.005%. Certainty with which the position of the electron can be located is
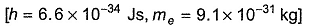
[AIEEE 2009]
The wavelength (in nanometer) associated with a proton (mass = 1.67 x 10-27 kg atom-1) at the velocity of 1.0 x 10-3 ms-1 is
Statement I : It is impossible to determine position and momentum of the moving electron simultaneously with same accuracy.
Statement II : The path of an electron in an atom is clearly defined.
If the photon of the wavelength 150 pm strikes an atom and one of its inner bound electrons is ejected out with a velocity of 1.5 x 107 ms-1, then binding energy by which electron is bound to nucleus is
The position of both, an electron and a helium atom is known within 1.0 mm. Further more the momentum of the electron is known within 5.0 x 10-26 kg ms-1. The minimum uncertainty in the measurement of the momentum of the helium atom is
The wavelength of a neutron with a translatory kinetic energy equal to kT at 300 K is
Direction (Q. Nos. 11 and 12) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)
Photoelectric effect can be expressed in terms of the following graph
Q. What is work function for this photoelectric emission of electrons?
|
360 tests
|