NEET Exam > NEET Tests > Topic-wise MCQ Tests for NEET > Test: Dual Nature of Matter - NEET MCQ
Test: Dual Nature of Matter - NEET MCQ
Test Description
10 Questions MCQ Test Topic-wise MCQ Tests for NEET - Test: Dual Nature of Matter
Test: Dual Nature of Matter for NEET 2025 is part of Topic-wise MCQ Tests for NEET preparation. The Test: Dual Nature of Matter questions and answers have been
prepared according to the NEET exam syllabus.The Test: Dual Nature of Matter MCQs are made for NEET 2025 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Dual Nature of Matter below.
Solutions of Test: Dual Nature of Matter questions in English are available as part of our Topic-wise MCQ Tests for NEET for NEET & Test: Dual Nature of Matter solutions in
Hindi for Topic-wise MCQ Tests for NEET course. Download more important topics, notes, lectures and mock
test series for NEET Exam by signing up for free. Attempt Test: Dual Nature of Matter | 10 questions in 10 minutes | Mock test for NEET preparation | Free important questions MCQ to study Topic-wise MCQ Tests for NEET for NEET Exam | Download free PDF with solutions
Test: Dual Nature of Matter - Question 1
A photon has energy of 2.6 eV. What is its wavelength?
Detailed Solution for Test: Dual Nature of Matter - Question 1
Test: Dual Nature of Matter - Question 2
If uncertainty in the position of an electron is zero, the uncertainity in its momentum would be
Detailed Solution for Test: Dual Nature of Matter - Question 2
Test: Dual Nature of Matter - Question 3
Uncertainty in the position of an electron (mass 9.1 x 10-31 kg) moving with a velocity of 300 ms-1, accurate upto 0.001% will be:
Detailed Solution for Test: Dual Nature of Matter - Question 3
Test: Dual Nature of Matter - Question 4
What experimental result did Albert Einstein cite as evidence that light has particle-like properties?
Detailed Solution for Test: Dual Nature of Matter - Question 4
Detailed Solution for Test: Dual Nature of Matter - Question 5
Test: Dual Nature of Matter - Question 6
The de – Broglie wavelength of an electron is 600 nm. The velocity of the electron having the mass 9.1 X 10-31 Kg is
Detailed Solution for Test: Dual Nature of Matter - Question 6
Detailed Solution for Test: Dual Nature of Matter - Question 7
Detailed Solution for Test: Dual Nature of Matter - Question 8
Test: Dual Nature of Matter - Question 9
Whch of the following will have the maximum value of ∆v . ∆x
Detailed Solution for Test: Dual Nature of Matter - Question 9
Test: Dual Nature of Matter - Question 10
The de Broglie wavelength of an electron is 8.7 x 10-11 m. The mass of an electron is 9.1 x 10-31 kg. The velocity of this electron is:
Detailed Solution for Test: Dual Nature of Matter - Question 10
|
9 docs|806 tests
|
Information about Test: Dual Nature of Matter Page
In this test you can find the Exam questions for Test: Dual Nature of Matter solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Dual Nature of Matter, EduRev gives you an ample number of Online tests for practice


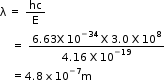

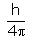 , ∆p is:
, ∆p is:










