Test: Dual Nature of Radiation & Matter - NEET MCQ
30 Questions MCQ Test Physics Class 12 - Test: Dual Nature of Radiation & Matter
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
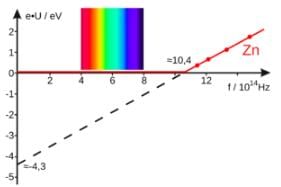
The work function of a photoelectric material is 3.32 eV. The threshold frequency will be equal to
Energy of a photon of green light of wavelength 5500m is (given: h = 6.62 ×10−34Js−1) approximately
Uniform electric and magnetic fields are produced pointing in the same direction. An electron is projected pointing in the same direction, then
In which case is electron emission from a metal not known?
Given h = 6.6 ×10−34 joule sec, the momentum of each photon in a given radiation is 3.3 ×10−29 kg metre/sec. The frequency of radiation is
If the work function of a material is 2eV, then minimum frequency of light required to emit photo-electrons is
In Thomson’s method for finding specific charge of positive rays, the electric and magnetic fields are
In a photon-particle collision (such as photon-electron collision) the quantity which is not conserved is
The specific charge for positive rays is much less than that for cathode rays. This is because
If the voltage across the electrodes of a cathode ray tube is 500 volts then energy gained by the electrons is
If work function of a metal plate is negligible then the K.E.of the photoelectrons emitted when radiations of 1000 Â are incident on the metal surface is
The maximum kinetic energy of photoelectrons emitted from a surface when photons of energy 6 eV fall on it is 4 eV. The stopping potential is
If maximum velocity with which an electron can be emitted from a photo cell is 3.75×108cms−1 then stopping potential is
In an experiment of photoelectric emission for incident light of 4000 Â, the stopping potential is 2V. If the wavelength of incident light is made 3000 Â, then stopping potential will be
In the above experimental set up for studying photoelectric effect, if keeping the frequency of the incident radiation and the accelerating potential fixed, the intensity of light is varied, then
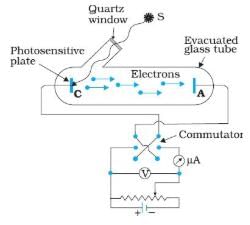
Photo-electric effect can be explained only by assuming that light
Wavelength of light incident on a photo cell is 3000 Â, if stopping potential is 2.5 volts, then work function of the cathode of photo cell is
in photoelectric effect, the photoelectric current
In various experiments on photo electricity the stopping potential for a given frequency of the incident radiation
In order to increase the kinetic energy of ejected photoelectrons, there should be an increase in
Each photon has the same speed but different
Number of ejected photoelectrons increases with increase
|
97 videos|336 docs|104 tests
|