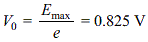Test: Dual Nature of Radiation and Matter - 1 - CUET Humanities MCQ
10 Questions MCQ Test Agriculture Practice Tests: CUET Preparation - Test: Dual Nature of Radiation and Matter - 1
In photoelectric effect, the electrons are ejected from metals if the incident light has a certain minimum
An X-ray tube produces a continuous spectrum of radiation with its short-wavelength end at 0.33Å. What is the maximum energy (Planck's constant = 6.6 x 10-34 Js) of a photon in the radiation?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
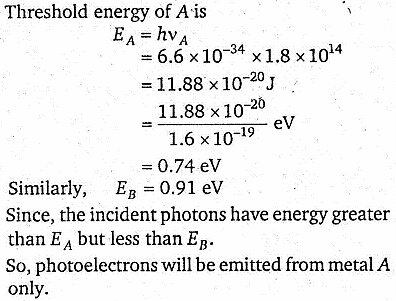
A and B are two metals with threshold frequencies 1.8 x 1014 Hz and 2.2 × 1014 Hz, respectively. Two identical photons of energy 0.825 eV are incident on them. Then, the photoelectrons (take h = 6.6 × 10-34 J-s) are emitted by
Consider the following two statements A and B, and identify the correct answer.
A: For electrons, the value of specific charge depends upon the gas taken in the tube.
B: Increase in the intensity of incident radiation in photoelectric emission increases the number of photoelectrons.
The photoelectric threshold for a certain metal surface is 330 Å. What is the maximum kinetic energy of a photoelectron released, if any, by a radiation of wavelength 1100 Å?
If the energy of a photon corresponding to a wavelength of 6000Å is 3.32 × 10-19 J, the photon energy for a wavelength of 4000 Å will be
When a light of wavelength 300 nm falls on a photoelectric emitter, the photoelectrons are just liberated. For another emitter, the light of wavelength 600 nm is sufficient for liberating photoelectrons. The ratio of the work function of the two emitters is
Ultraviolet radiation of energy 6.2 eV falls on the surface of aluminium of work function 4.2 eV. What will be the K.E. of the fastest electron (in joule)?
A metal whose work function is 3.3 eV is illuminated by light of wavelength 3 × 10-7 m. What is the stopping potential? (Planck's constant = 6.6 × 10-34 Js)
The photoelectric effect is based on the law of conservation of


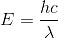
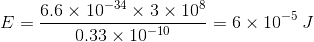
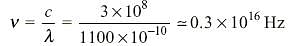

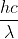
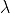 is wavelength.
is wavelength.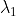 = 6000
= 6000 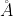 , E1 = 3.32 × 10-19 J,
, E1 = 3.32 × 10-19 J, 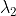 = 4000
= 4000 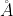 .
.

 × 3.32 × 10-19 J
× 3.32 × 10-19 J eV = 3.1 eV
eV = 3.1 eV
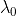 is the threshold wavelength.
is the threshold wavelength.